
Concept explainers
Synthesize each compound from toluene
a.
b.
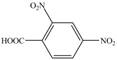 f.
f.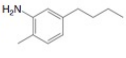
c.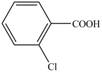 e.
e. 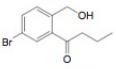 g.
g.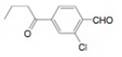
(a)
Interpretation: The synthesis of given compound from toluene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.66P
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below:
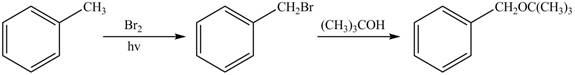
Explanation of Solution
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below.
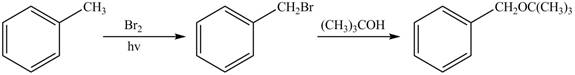
Figure 1
The first step of the synthesis is monobromination of methyl group of toluene in presence of light. The product obtained is treated with tertiary butanol to form desired ether.
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 1.
(b)
Interpretation: The synthesis of given compound from toluene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.66P
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below:
Explanation of Solution
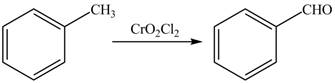
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 1.
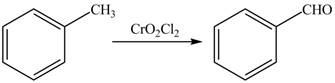
Figure 2
Toluene undergoes Etard’s reaction using inorganic reagent
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 2.
(c)
Interpretation: The synthesis of given compound from toluene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.66P
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below:
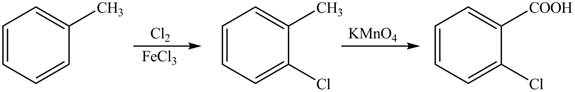
Explanation of Solution
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below.
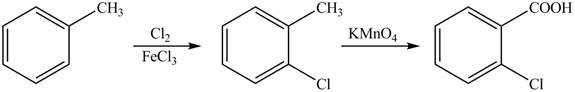
Figure 3
Toluene undergoes chlorination on ortho and para position, but for the synthesis ortho product is required. This ortho product is oxidized using potassium permanganate to form the desired product.
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 3.
(d)
Interpretation: The synthesis of given compound from toluene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.66P
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below:
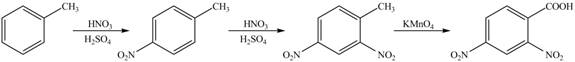
Explanation of Solution
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below.
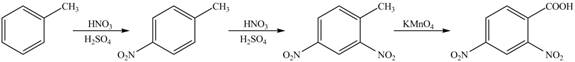
Figure 4
Toluene undergoes nitration on ortho and para position, but for the synthesis para product is required. This product again undergoes nitration on the meta position to nitro product. This product is oxidized using potassium permanganate to form the desired product.
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 4.
(e)
Interpretation: The synthesis of given compound from toluene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.66P
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below:
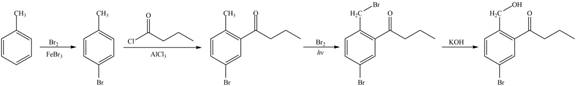
Explanation of Solution
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below.
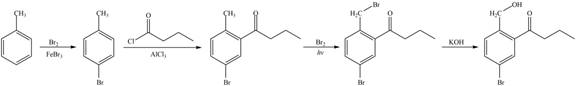
Figure 5
The first step of the synthesis is bromination of toluene which is followed by Friedel-Craft acylation. The product obtained by the Friedel-Craft acylation undergoes bromination at allylic carbon atom and on further reaction with strong base bromine group is replaced by hydroxyl group. This leads to the formation of desired product.
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 5.
(f)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.66P
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below:
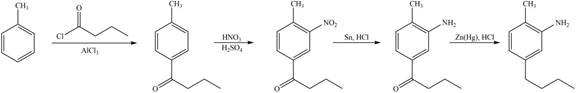
Explanation of Solution
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below.
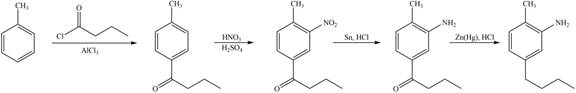
Figure 6
The first step of the synthesis is Friedel-Craft acylation of toluene which is followed by nitration reaction. The product obtained by nitration undergoes reduction in presence of
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 6.
(g)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.66P
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below:
Explanation of Solution
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown below.
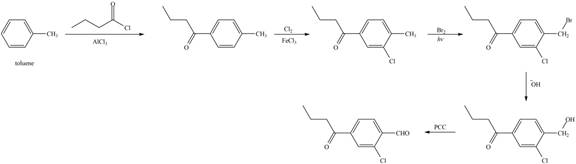
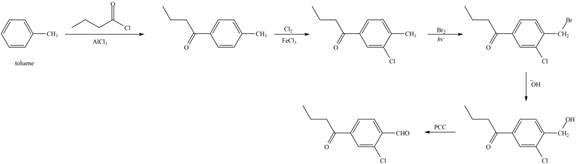
Figure 7
The first step of the synthesis is Friedel-Craft acylation of toluene which is followed by chlorination. The product obtained by chlorination undergoes bromination at allylic carbon atom and on further reaction with strong base bromine group is replaced by hydroxyl group. The
The synthesis of given compound from toluene by the use of organic and inorganic reagent is shown in Figure 7.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry-Package(Custom)
- To answer the following questions, consider the reaction below: CH3 . CH3 OH a. The best reagents for accomplishing the above transformation are.... a. 1. OsO4, pyridine 2. NaHSO3, H₂O b. 1. Hg(OAc)2, H₂O 1. C. 2. NaBH4 RCO₂H, CH2Cl₂ 2. H₂O* d. 1. BH3, THF 2. H₂O₂, OH b. The alcohol product is classified as a: a. 1° alcohol b. 2° alcohol C. 3° alcohol d. 4° alcohol c. The conversion of an alcohol into an alkyl chloride by reaction with SOCI2 is an example of: a. b. ن نخنه C. d. an El process an Syl process an E2 process an Sy2 processarrow_forwardEstimation of ash in food Questions: Q1: What does the word ash refer to? Q2: Mention the types of ash in food Q3: Mention the benefit of using a glass dryerarrow_forwardDraw structures corresponding to the names given a. m-fluoronitrobenzene b. p-bromoaniline c. o-chlorophenol d. 3,5-dimethylbenzoic acidarrow_forward
- Illustrate the reaction mechanism the following reactionarrow_forwardPropose a synthesis for the following compound using benzene or toluene and any other reagents necessary. Show all major intermediate compounds that would probably be isolated during the course of your synthesis. on. Harrow_forwardProvide correct IUPAC names for each of the following compounds. NOT a. b. C. 2003 H,N- CH3 NH2 CHarrow_forward
- . Consider the reaction below to answer the following questions. OH 1. NaH 2. CH3I, ether O-CH3 A. Write the complete stepwise mechanism for the reaction. Show all intermediate structures and all electron flow with arrows. B. Mechanistically, the Williamson ether synthesis outlined above is: ن نخنه a. an El process b. an SN1 process C. an E2 process d. an SN2 process C. Alternatively, cyclopentyl methyl ether may be synthesized from cyclopentene. synthesis of cyclopentyl methyl ether from cyclopentene. Outline aarrow_forwardQ2. A good synthesis of (CH3)3C- would be: A) B) CSI3 0 CH3CC1 (CH3) 3CC1 Benzene AlCl3 AlCl3 (CH3)3CC1 CH3CC1 Benzene C) AlCl3 0 AlCl3 CH3CC1 (CH3) 2C-CH2 Bonzone AlCl3 HF D) More than one of these E) None of thesearrow_forwardDon't used hand raiting and correct answer and don't used Ai solutionarrow_forward
- Show how you might carry out the following transformation or reactions: toluene to m-chlorobenzoic acidarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardCan you please explain how to solve this problem step by step? You might consider color coding it or presenting it in a way that makes it easier for me to understand.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
