
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.65P
Synthesize each compound from benzene and any other organic or inorganic reagents.
a. d
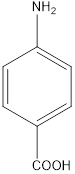
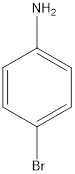
b. e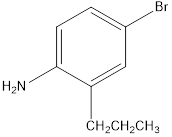
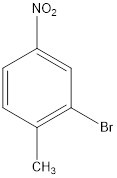 .
.
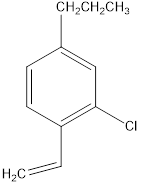 c. f.
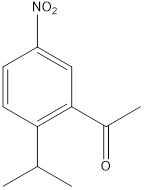
c. f.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
Don't used hand raiting and don't used Ai solution
Q3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2
reaction rate.
CI
Cl
H3C-Cl
CI
a)
A
B
C
D
Br
Br
b)
A
B
C
Br
H3C-Br
D
Chapter 18 Solutions
Organic Chemistry-Package(Custom)
Ch. 18 - Prob. 18.1PCh. 18 - Prob. 18.2PCh. 18 - Prob. 18.3PCh. 18 - Prob. 18.4PCh. 18 - Prob. 18.5PCh. 18 - What acid chloride would be needed to prepare each...Ch. 18 - Prob. 18.7PCh. 18 - Draw a stepwise mechanism for the following...Ch. 18 - Prob. 18.9PCh. 18 - Prob. 18.10P
Ch. 18 - Prob. 18.11PCh. 18 - Prob. 18.12PCh. 18 - Problem 18.14 Draw all resonance structures for...Ch. 18 - Classify each substituent as electron donating or...Ch. 18 - Prob. 18.15PCh. 18 - Label each compound as more or less reactive than...Ch. 18 - Rank the following compounds in order of...Ch. 18 - Problem 18.18 Rank the following compounds in...Ch. 18 - Prob. 18.19PCh. 18 - Problem 18.20 Draw the products of each...Ch. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - Devise a synthesis of each compound from the...Ch. 18 - Problem 18.24 Draw the products of each...Ch. 18 - Problem 18.25 Draw a stepwise mechanism for the...Ch. 18 - Problem 18.26 Draw the products of each...Ch. 18 - Prob. 18.27PCh. 18 - Prob. 18.28PCh. 18 - Problem 18.29 How could you use ethylbenzene to...Ch. 18 - Prob. 18.30PCh. 18 - Problem 18.31 What steps are needed to convert...Ch. 18 - Problem 18.32 Synthesize each compound from...Ch. 18 - Synthesize each compound from benzene.Ch. 18 - Prob. 18.34PCh. 18 - 18.35 What is the major product formed by an...Ch. 18 - Draw the products formed when phenol (C6H5OH) is...Ch. 18 - Prob. 18.37PCh. 18 - Draw the products of each reaction. a. e. i. b. f....Ch. 18 - What products are formed when benzene is treated...Ch. 18 - Draw the products of each reaction. a.d. b.e. c.f.Ch. 18 - You have learned two ways to make an alkyl...Ch. 18 - Prob. 18.42PCh. 18 - Prob. 18.43PCh. 18 - 18.45 Explain why each of the following reactions...Ch. 18 - Prob. 18.45PCh. 18 - 18.47 For each of the following substituted...Ch. 18 - Which benzene ring in each compound is more...Ch. 18 - 18.49 For each N-substituted benzene, predict...Ch. 18 - Explain each statement in detail using resonance...Ch. 18 - Prob. 18.50PCh. 18 - Explain why the meta product is formed in the...Ch. 18 - 18.53 Rank the aryl halides in each group in order...Ch. 18 - Prob. 18.53PCh. 18 - 18.54 Draw a stepwise mechanism for the following...Ch. 18 - Prob. 18.55PCh. 18 - 18.56 Draw a stepwise, detailed mechanism for the...Ch. 18 - Prob. 18.57PCh. 18 - 18.58 Draw a stepwise mechanism for the following...Ch. 18 - Draw a stepwise mechanism for the following...Ch. 18 - Prob. 18.60PCh. 18 - Draw a stepwise mechanism for the following...Ch. 18 - Prob. 18.62PCh. 18 - Prob. 18.63PCh. 18 - Synthesize each compound from benzene and any...Ch. 18 - Synthesize each compound from benzene and any...Ch. 18 - Synthesize each compound from toluene (C6H5CH3)...Ch. 18 - Prob. 18.67PCh. 18 - Use the reactions in this chapter along with those...Ch. 18 - Prob. 18.69PCh. 18 - Prob. 18.70PCh. 18 - Problem 18.69 Identify the structures of isomers A...Ch. 18 - Prob. 18.72PCh. 18 - Problem 18.71 Compound X (molecular formula ) was...Ch. 18 - Prob. 18.74PCh. 18 - The NMR spectrum of phenol () shows three...Ch. 18 - Explain the reactivity and orientation effects...Ch. 18 - Prob. 18.77PCh. 18 - Prob. 18.78PCh. 18 - Prob. 18.79PCh. 18 - Prob. 18.80P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forward10. The main product of the following reaction is [1.1:4',1"-terphenyl]-2'-yl(1h-pyrazol-4- yl)methanone Ph N-H Pharrow_forwardDraw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total stereoisomers are there? Name the sugar you drew. Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total stereoisomers are there? Draw the enantiomer.arrow_forward
- Draw a structure using wedges and dashes for the following compound: H- Et OH HO- H H- Me OHarrow_forwardWhich of the following molecules are NOT typical carbohydrates? For the molecules that are carbohydrates, label them as an aldose or ketose. HO Он ОН ОН Он ОН но ΤΗ HO ОН HO eve Он он ОН ОН ОН If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units are present?arrow_forwardDraw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forward
- Name the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forwardWhat are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY