
Concept explainers
Synthesize each compound from benzene and any other organic or inorganic reagents.
a.
b.
c. 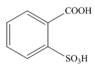 d.
d. 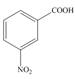 e.
e. ![]() f.
f. 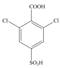 g.
g. 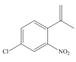
(a)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
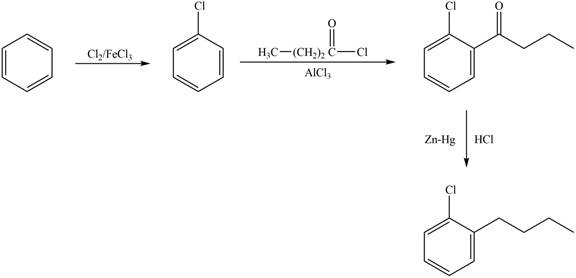
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 1.
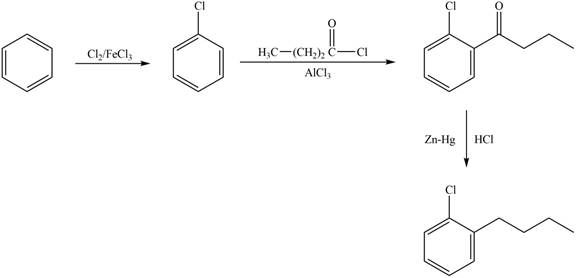
Figure 1
The first step in the synthesis is the chlorination of benzene. The next step is the Friedel-Craft acylation in which chlorobenzene reacts with butnoyl chloride. The last step is the clemmensen reduction to form the desired product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 1.
(b)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
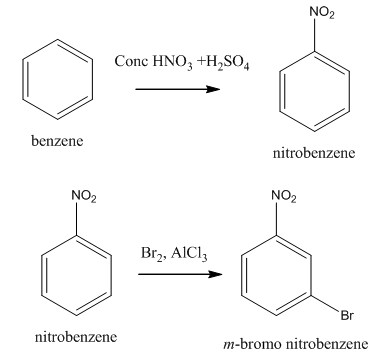
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 2.

Figure 2
The first step in the synthesis is the formation of nitrobenzene. The next step is the reaction of nitrobenzene with bromine in the presence of aluminum chloride to give the final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 2.
(c)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below.
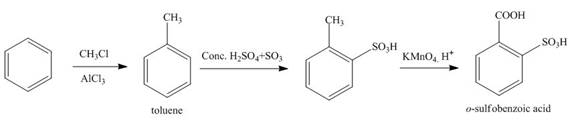
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.

Figure 3
The first step in the synthesis is the conversion of benzene into toluene. The next step is the reaction of toluene with concentrated sulfuric acid which results the addition of sulphonyl group at ortho position. The last step is the oxidation of methyl group into acid in the presence of potassium permanganate to give the final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.
(d)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
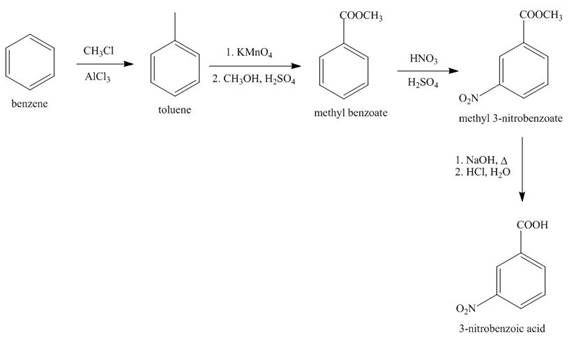
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 4.

Figure 4
The first step in the synthesis is the conversion of benzene into toluene. The next step is the oxidation of methyl group into acid in the presence of potassium permanganate to give benzoic acid followed by the reaction with methanol to form methyl benzoate. The reaction of nitric acid in the presence of sulphuric acid results in the formation of final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 4.
(e)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
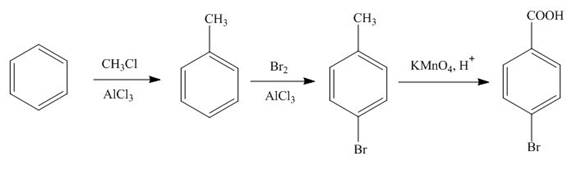
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 5.
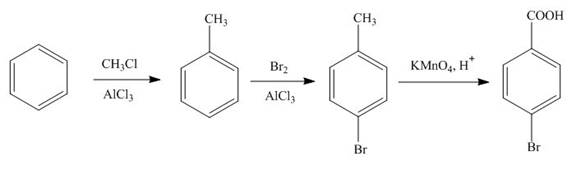
Figure 5
The first step in the synthesis is the conversion of benzene into toluene. The next step is the reaction of toluene with bromine in the presence of aluminum chloride. The last step is the oxidation of methyl group into acid in the presence of potassium permanganate to give the final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 5.
(f)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
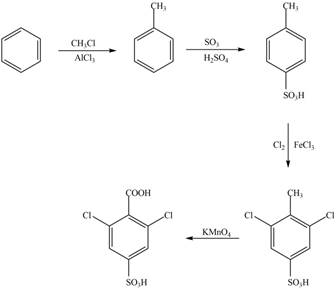
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 6.

Figure 6
The synthesis of given compound take place in four steps: Friedel-Craft alkylation, sulfonation, chlorination and at last oxidation.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 6.
(g)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
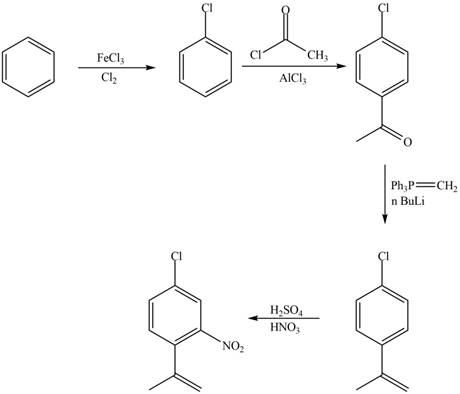
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.
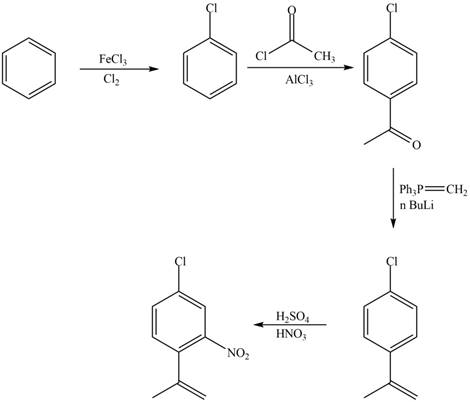
Figure 7
The first, second, third and fourth step involved in the synthesis of given compound is chlorination, Friedel-Craft acylation, Wittig reaction and nitration, respectively.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 7.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry-Package(Custom)
- The number of hydrogens in an alkyne that has a main chain of 14carbons to which are attached a cyclobutyl ring, a benzene ring, an–OH group, and a Br is A. 34; B. 35; C. 36; D. 24; E. 43arrow_forwardHello! I have a 500 Hz H-NMR for 1,5-bis-(4-methoxyphenyl)-penta-1,4-dien-3-one. I need to label the signals with the corresponding H's. Then, find out if the two alkenes are cis or trans by calculating the J values. I believe that I have the H-NMR labeled correctly, but not sure if I got the J values correct to determine if the two alkenes in the compound will make the compound cis or trans.arrow_forwardWhat is the only possible H-Sb-H bond angle in SbH3?arrow_forward
- Predict the product formed when the compound shown below undergoes a reaction with MCPBA in CH2Cl2. MCPBA is meta-chloroperoxybenzoic acid.arrow_forwardk https://app.aktiv.com STARTING AMOUNT 6 58°F Clear + F1 X Dimensional Analysis - Aktiv Chemistry Your Aktiv Learning trial expires on 02/25/25 at 02:14 PM Question 19 of 22 Polyethylene terephthalate (PET) is used in plastic water bottles. A water bottle has a mass of 14.0 grams. Given a density of 1.38 g/cm³, what is the volume of the plastic used to make the water bottle in cm³ ? ADD FACTOR ANSWER RESET ว 100 14.0 0.01 10.1 1000 0.099 1.38 0.001 Q Search F5 -O+ F6 F7 + F3 F2 W E S4 ST #3 F4 % 5 Y R S & 7 cm³ g/cm³ g ם F8 * 00 8 F9 P ل DOD S F10 F11 F12 Insert D F G H J K + 11arrow_forwardA doctor gives a patient 10 Ci of beta radiation. How many betaparticles would the patient receive in 1 minute? (1 Ci = 3.7 x 1010d/s)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





