
Concept explainers
Permethrin and Bifenthrin
Pyrethrin is a natural insecticide obtained from the powdered flower heads of several species of Chrysanthemum. The active substances in pyrethrum, principally pyrethrins I and II, are contact poisons for insects and cold-blooded vertebrates. Although powders made from Chrysanthemum extracts have found widespread use, the active substances in them are destroyed rapidly in the environment. In an effort to develop synthetic compounds as effective as the natural insecticides but with greater biostability, chemists prepared a series of esters related in structure. Among the synthetic pyrethrenoids now in common use in household and agricultural products are permethrin and bifenthrin.
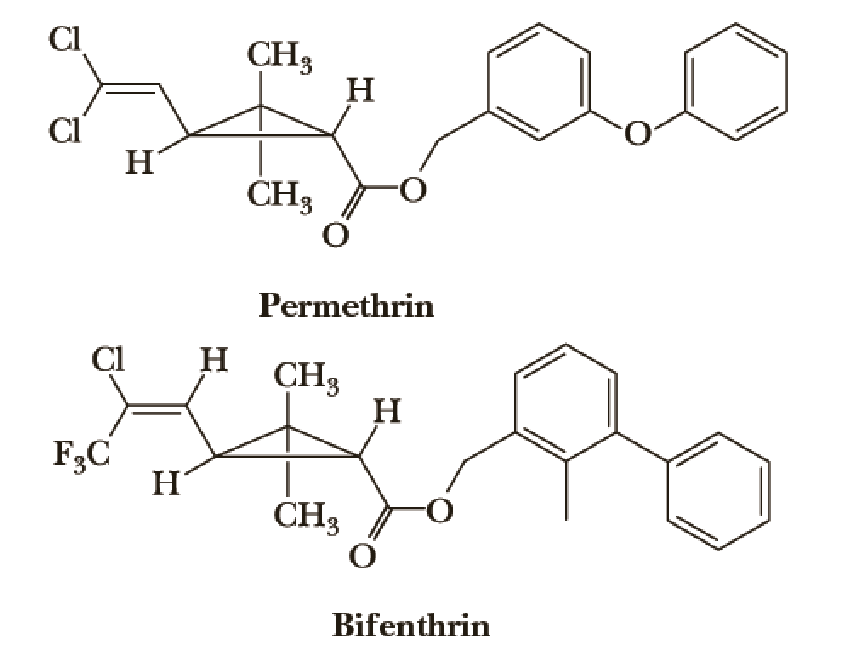
As discussed above, the natural products pyrethrins I and II (not shown) are destroyed rapidly in the environment. One of the key changes in the structures of permethrin and bifenthrin relative to the pyrethrins was the substitution of naturally occurring methyl groups on the
- 1. Oxidation of the double bond.
- 2. Electrophilic addition reactions.
- 3. Nucleophilic addition reactions.
- 4. Both 1 and 2.
- 5. All of the above.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry
- (ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward(ME EX1) Prblm #4-11 Can you please help me and explain these I'm very confused in detail please. Prblm number 9 I don't understand at all (its soo confusing to me and redraw it so I can better depict it).arrow_forward
- ME EX1) Prblm #19-20 I'm so confused with these problems. Can you please help me solve them and explain them? Problems number 19-20, and thanks! step by step and in detail for me please helparrow_forwardCalculate the flux of oxygen between the ocean and the atmosphere, given that: Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturatedarrow_forward( ME EX1) Prblm 27-28: Can you explain to me both prblms in detail and for prblm 28 what do you mean bi conjugated bi ponds and those structures I'm confused...arrow_forward
- A. Determine the number of electrons in a system of cyclic conjugation (zero if no cyclic conjugation). B. Specify whether the species is "a"-aromatic, "aa"-anti-aromatic, or "na"-non-aromatic (neither aromatic nor anti-aromatic). (Presume rings to be planar unless structure obviously prevents planarity. If there is more than one conjugated ring, count electrons in the largest.) 1. A.Electrons in a cyclic conjugated system. 18 B.The compound is (a, aa, or na) a 2. A.Electrons in a cyclic conjugated system. 10 B.The compound is (a, aa, or na) naarrow_forwardWater is boiling at 1 atm pressure in a stainless steel pan on an electric range. It is observed that 2 kg of liquid water evaporates in 30 min. Find the rate of heat transfer to the water (kW).arrow_forwardCould you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the resonance structures that were given please.arrow_forward
- Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the question.arrow_forwardplease solve. If the answer is "no error" and it asks me to type something, and i typed a-helix, its always wrong.arrow_forwardCan you please solve and explain this for me in a simple way? I cant seem to comprehend this problem.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning




