
(a)
Interpretation: The name of
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,
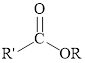
Fischer esterification can be represented as follows,
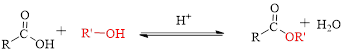
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
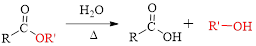
(b)
Interpretation: The name of carboxylic acid and alcohol has to be determined, from which the given eseter is derived.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,

Fischer esterification can be represented as follows,
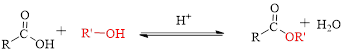
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
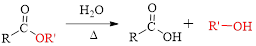
(c)
Interpretation: The name of carboxylic acid and alcohol has to be determined, from which the given eseter is derived.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,

Fischer esterification can be represented as follows,
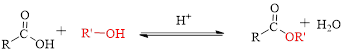
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
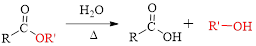
(d)
Interpretation: The name of carboxylic acid and alcohol has to be determined, from which the given eseter is derived.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology.
In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuroc acid.
Esters can be simply represented as,

Fischer esterification can be represented as follows,
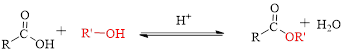
The splitting of an ester into the component acid and alcohol is known as ester hydrolysis.
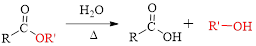
Trending nowThis is a popular solution!

Chapter 17 Solutions
Organic Chemistry
- TRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forward
- In the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forwardUsing spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forwardCalculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forward
- The Ka for sodium dihydrogen phosphate is 6.32 x 10-8. Find the pH of a buffer made from 0.15 M H2PO4- and 0.25 M HPO42- .arrow_forwardThe Ka for lactic acid is 1.4 x 10-4. Find the pH of a buffer made from 0.066 M lactic acid and 0.088 M sodium lactate.arrow_forwardZaitsev's Rule 3) (a) Rank the following alkenes in order of decreasing stability. most stable A B C D > > > (b) Rank the following carbocations in order of decreasing stability least stable B C Darrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





