
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 17.27P
Low-molecular-weight dicarboxylic acids normally exhibit two different pKa values. Ionization of the first carboxyl group is easier than the second. This effect diminishes with molecular size, and for adipic acid and longer chain dicarboxylic acids, the two acid ionization constants differ by about one pK unit.
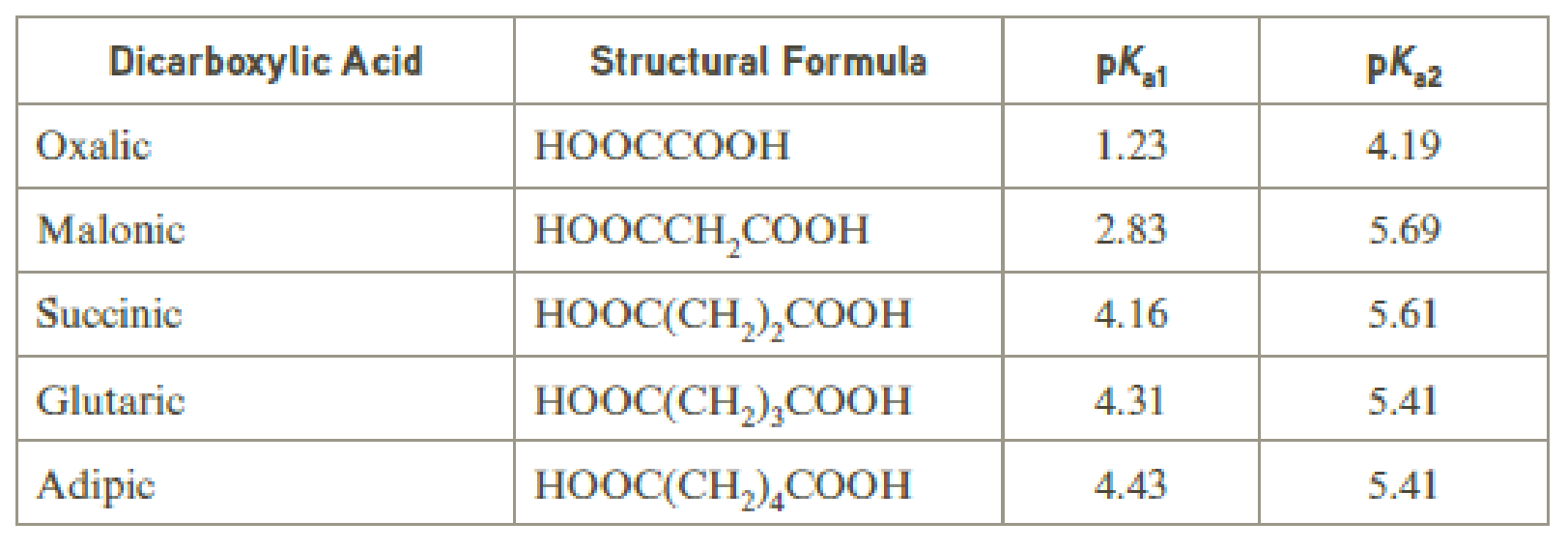
Why do the two pKa values differ more for the shorter chain dicarboxylic acids than for the longer chain dicarboxylic acids?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 17 Solutions
Organic Chemistry
Ch. 17.2 - Prob. 17.1PCh. 17.4 - Which is the stronger acid in each pair?Ch. 17.4 - Prob. 17.3PCh. 17.7 - Prob. 17.4PCh. 17.8 - Prob. 17.5PCh. 17.8 - Prob. AQCh. 17.8 - Prob. BQCh. 17.8 - Prob. CQCh. 17.8 - Permethrin and Bifenthrin Pyrethrin is a natural...Ch. 17.9 - Prob. 17.6P
Ch. 17 - Write the IUPAC name of each compound, showing...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10PCh. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - On a cyclohexane ring, an axial carboxyl group has...Ch. 17 - Prob. 17.15PCh. 17 - Prob. 17.16PCh. 17 - Prob. 17.17PCh. 17 - Complete each reaction.Ch. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Show the reagents and experimental conditions...Ch. 17 - Prob. 17.23PCh. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - In each set, assign the acid its appropriate pKa.Ch. 17 - Low-molecular-weight dicarboxylic acids normally...Ch. 17 - Complete the following acid-base reactions. (a)...Ch. 17 - Prob. 17.29PCh. 17 - Prob. 17.30PCh. 17 - Excess ascorbic acid is excreted in the urine, the...Ch. 17 - Give the expected organic product when...Ch. 17 - Show how to convert trans-3-phenyl-2-propenoic...Ch. 17 - Show how to convert 3-oxobutanoic acid...Ch. 17 - Prob. 17.35PCh. 17 - Prob. 17.36PCh. 17 - Prob. 17.37PCh. 17 - When 4-hydroxybutanoic acid is treated with an...Ch. 17 - Fischer esterification cannot be used to prepare...Ch. 17 - Draw the product formed on thermal decarboxylation...Ch. 17 - Prob. 17.41PCh. 17 - Show how cyclohexanecarboxylic acid could be...Ch. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Write the products of the following sequences of...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Prob. 17.51PCh. 17 - Complete the following Fischer esterification...Ch. 17 - Prob. 17.53P
Additional Science Textbook Solutions
Find more solutions based on key concepts
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals of Physics Extended
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License