
Concept explainers
Interpretation:
A
Concept Introduction:
Carboxylic acid: One
Ester: One
Esterification reaction: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
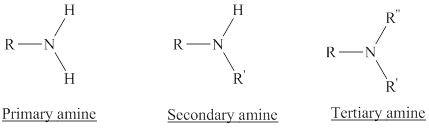
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
Aromatic Compounds: Compounds that are planar, conjugated, cyclic and having
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- please draw it for me and tell me where i need to modify the structurearrow_forwardPlease help determine the standard curve for my Kinase Activity in Excel Spreadsheet. Link: https://mnscu-my.sharepoint.com/personal/vi2163ss_go_minnstate_edu/_layouts/15/Doc.aspx?sourcedoc=%7B958f5aee-aabd-45d7-9f7e-380002892ee0%7D&action=default&slrid=9b178ea1-b025-8000-6e3f-1cbfb0aaef90&originalPath=aHR0cHM6Ly9tbnNjdS1teS5zaGFyZXBvaW50LmNvbS86eDovZy9wZXJzb25hbC92aTIxNjNzc19nb19taW5uc3RhdGVfZWR1L0VlNWFqNVc5cXRkRm4zNDRBQUtKTHVBQldtcEtWSUdNVmtJMkoxQzl3dmtPVlE_cnRpbWU9eEE2X291ZHIzVWc&CID=e2126631-9922-4cc5-b5d3-54c7007a756f&_SRM=0:G:93 Determine the amount of VRK1 is present 1. Average the data and calculate the mean absorbance for each concentration/dilution (Please over look for Corrections) 2. Blank Correction à Subtract 0 ug/mL blank absorbance from all readings (Please over look for Corrections) 3. Plot the Standard Curve (Please over look for Corrections) 4. Convert VRK1 concentration from ug/mL to g/L 5. Use the molar mass of VRK1 to convert to M and uM…arrow_forwardMacmillan Learning Cholesterol synthesis begins with the formation of mevalonate from acetyl CoA. This process activates mevalonate and converts it to isopentenyl pyrophosphate. Identify the atoms in mevalonate and isopentenyl pyrophosphate that will be labeled from acetyl CoA labeled with 14C in the carbonyl carbon. Place 14C atoms and C atoms to denote which carbon atoms are labeled and which are not labeled. H₂C COA 14C-labeled acetyl-CoA HHH [c] H H OH 014C - OH H HH H Mevalonate CH3 H H 14C H Η H H Incorrect Answer of o -P-O-P-0- Isopentenyl pyrophosphate с Answer Bank 14Carrow_forward
- Draw the reaction between sphingosine and arachidonic acid. Draw out the full structures.arrow_forwardDraw both cis and trans oleic acid. Explain why cis-oleic acid has a melting point of 13.4°C and trans-oleic acid has a melting point of 44.5°C.arrow_forwardDraw the full structure of the mixed triacylglycerol formed by the reaction of glycerol and the fatty acids arachidic, lauric and trans-palmitoleic. Draw the line structure.arrow_forward
- Draw out the structure for lycopene and label each isoprene unit. "Where is lycopene found in nature and what health benefits does it provide?arrow_forwardWhat does it mean to be an essential fatty acid? What are the essential fatty acids?arrow_forwardCompare and contrast primary and secondary active transport mechanisms in terms of energy utilisation and efficiency. Provide examples of each and discuss their physiological significance in maintaining ionic balance and nutrient uptake. Rubric Understanding the key concepts (clearly and accurately explains primary and secondary active transport mechanisms, showing a deep understanding of their roles) Energy utilisation analysis ( thoroughly compares energy utilisation in primary and secondary transport with specific and relevant examples Efficiency discussion Use of examples (provides relevant and accurate examples (e.g sodium potassium pump, SGLT1) with clear links to physiological significance. Clarity and structure (presents ideas logically and cohesively with clear organisation and smooth transition between sections)arrow_forward
- 9. Which one of the compounds below is the major organic product obtained from the following reaction sequence, starting with ethyl acetoacetate? 요요. 1. NaOCH2CH3 CH3CH2OH 1. NaOH, H₂O 2. H3O+ 3. A OCH2CH3 2. ethyl acetoacetate ii A 3. H3O+ OH B C D Earrow_forward7. Only one of the following ketones cannot be made via an acetoacetic ester synthesis. Which one is it? Ph کہ A B C D Earrow_forward2. Which one is the major organic product obtained from the following reaction sequence? HO A OH 1. NaOEt, EtOH 1. LiAlH4 EtO OEt 2. H3O+ 2. H3O+ OH B OH OH C -OH HO -OH OH D E .CO₂Etarrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





