
Bundle: Chemistry: An Atoms First Approach, 2nd, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
2nd Edition
ISBN: 9781305717633
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 50E
The amount of manganese in steel is determined by changing it to permanganate ion. The steel is first dissolved in nitric acid, producing Mn2+ ions. These ions are then oxidized to the deeply colored MnO4− ions by periodate ion (IO4−) in acid solution.
a. Complete and balance an equation describing each of the above reactions.
b. Calculate 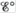 and ΔG° at 25°C for each reaction.
and ΔG° at 25°C for each reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 17 Solutions
Bundle: Chemistry: An Atoms First Approach, 2nd, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 17 - What is a half-reaction? Why must the number of...Ch. 17 - Galvanic cells harness spontaneous...Ch. 17 - Table 17-1 lists common half-reactions along with...Ch. 17 - Prob. 4RQCh. 17 - The Nernst equation allows determination of the...Ch. 17 - Prob. 6RQCh. 17 - Prob. 7RQCh. 17 - Prob. 8RQCh. 17 - What characterizes an electrolytic cell? What is...Ch. 17 - Sketch a galvanic cell, and explain how it works....
Ch. 17 - Prob. 2ALQCh. 17 - Prob. 3ALQCh. 17 - Prob. 4ALQCh. 17 - Sketch a cell that forms iron metal from iron(II)...Ch. 17 - Which of the following is the best reducing agent:...Ch. 17 - Prob. 7ALQCh. 17 - Prob. 8ALQCh. 17 - Explain why cell potentials are not multiplied by...Ch. 17 - What is the difference between and ? When is equal...Ch. 17 - Prob. 11ALQCh. 17 - Look up the reduction potential for Fe3+ to Fe2+....Ch. 17 - Prob. 13ALQCh. 17 - Is the following statement true or false?...Ch. 17 - Prob. 15RORRCh. 17 - Assign oxidation numbers to all the atoms in each...Ch. 17 - Specify which of the following equations represent...Ch. 17 - The Ostwald process for the commercial production...Ch. 17 - Prob. 19QCh. 17 - Prob. 20QCh. 17 - When magnesium metal is added to a beaker of...Ch. 17 - How can one construct a galvanic cell from two...Ch. 17 - The free energy change for a reaction, G, is an...Ch. 17 - What is wrong with the following statement: The...Ch. 17 - When jump-starting a car with a dead battery, the...Ch. 17 - Prob. 26QCh. 17 - Prob. 27QCh. 17 - Consider the following electrochemical cell: a. If...Ch. 17 - Balance the following oxidationreduction reactions...Ch. 17 - Prob. 30ECh. 17 - Prob. 31ECh. 17 - Prob. 32ECh. 17 - Chlorine gas was first prepared in 1774 by C. W....Ch. 17 - Gold metal will not dissolve in either...Ch. 17 - Prob. 35ECh. 17 - Consider the following galvanic cell: a. Label the...Ch. 17 - Prob. 37ECh. 17 - Sketch the galvanic cells based on the following...Ch. 17 - Prob. 39ECh. 17 - Prob. 40ECh. 17 - Prob. 41ECh. 17 - Prob. 42ECh. 17 - Prob. 43ECh. 17 - Give the standard line notation for each cell in...Ch. 17 - Prob. 45ECh. 17 - Prob. 46ECh. 17 - Prob. 47ECh. 17 - Prob. 48ECh. 17 - Prob. 49ECh. 17 - The amount of manganese in steel is determined by...Ch. 17 - Prob. 51ECh. 17 - Prob. 52ECh. 17 - Estimate for the half-reaction 2H2O+2eH2+2OH given...Ch. 17 - Prob. 54ECh. 17 - Glucose is the major fuel for most living cells....Ch. 17 - Direct methanol fuel cells (DMFCs) have shown some...Ch. 17 - Prob. 57ECh. 17 - Using data from Table 17-1, place the following in...Ch. 17 - Answer the following questions using data from...Ch. 17 - Prob. 60ECh. 17 - Consider only the species (at standard conditions)...Ch. 17 - Prob. 62ECh. 17 - Prob. 63ECh. 17 - Prob. 64ECh. 17 - Prob. 65ECh. 17 - Prob. 66ECh. 17 - A galvanic cell is based on the following...Ch. 17 - Prob. 68ECh. 17 - Consider the concentration cell shown below....Ch. 17 - Prob. 70ECh. 17 - The overall reaction in the lead storage battery...Ch. 17 - Prob. 72ECh. 17 - Consider the cell described below:...Ch. 17 - Consider the cell described below:...Ch. 17 - Prob. 75ECh. 17 - Prob. 76ECh. 17 - Prob. 77ECh. 17 - Prob. 78ECh. 17 - Prob. 79ECh. 17 - An electrochemical cell consists of a nickel metal...Ch. 17 - An electrochemical cell consists of a standard...Ch. 17 - Prob. 82ECh. 17 - Consider a concentration cell that has both...Ch. 17 - Prob. 84ECh. 17 - Prob. 85ECh. 17 - Prob. 86ECh. 17 - Consider the following galvanic cell at 25C:...Ch. 17 - Prob. 88ECh. 17 - Prob. 89ECh. 17 - Prob. 90ECh. 17 - Prob. 91ECh. 17 - The solubility product for CuI(s) is 1.1 102...Ch. 17 - How long will it take to plate out each of the...Ch. 17 - The electrolysis of BiO+ produces pure bismuth....Ch. 17 - What mass of each of the following substances can...Ch. 17 - Prob. 96ECh. 17 - An unknown metal M is electrolyzed. It took 74.1 s...Ch. 17 - Electrolysis of an alkaline earth metal chloride...Ch. 17 - What volume of F2 gas, at 25C and 1.00 atm, is...Ch. 17 - What volumes of H2(g) and O2(g) at STP are...Ch. 17 - Prob. 101ECh. 17 - A factory wants to produce 1.00 103 kg barium...Ch. 17 - It took 2.30 min using a current of 2.00 A to...Ch. 17 - A solution containing Pt4+ is electrolyzed with a...Ch. 17 - A solution at 25C contains 1.0 M Cd2+, 1.0 M Ag+,...Ch. 17 - Consider the following half-reactions: A...Ch. 17 - In the electrolysis of an aqueous solution of...Ch. 17 - Copper can be plated onto a spoon by placing the...Ch. 17 - Prob. 109ECh. 17 - Prob. 110ECh. 17 - Prob. 111ECh. 17 - What reaction will take place at the Cathode and...Ch. 17 - Gold is produced electrochemically from an aqueous...Ch. 17 - Prob. 114AECh. 17 - The saturated calomel electrode. abbreviated SCE....Ch. 17 - Consider the following half-reactions: Explain why...Ch. 17 - Consider the standard galvanic cell based on the...Ch. 17 - Prob. 118AECh. 17 - The black silver sulfide discoloration of...Ch. 17 - Prob. 120AECh. 17 - When aluminum foil is placed in hydrochloric acid,...Ch. 17 - Prob. 122AECh. 17 - Prob. 123AECh. 17 - The overall reaction and equilibrium constant...Ch. 17 - What is the maximum work that can be obtained from...Ch. 17 - The overall reaction and standard cell potential...Ch. 17 - Prob. 127AECh. 17 - Prob. 128AECh. 17 - Prob. 129AECh. 17 - Prob. 130AECh. 17 - Prob. 131AECh. 17 - Prob. 132AECh. 17 - Prob. 133AECh. 17 - Prob. 134CWPCh. 17 - Consider a galvanic cell based on the following...Ch. 17 - Consider a galvanic cell based on the following...Ch. 17 - Consider a galvanic cell based on the following...Ch. 17 - An electrochemical cell consists of a silver metal...Ch. 17 - An aqueous solution of PdCl2 is electrolyzed for...Ch. 17 - Prob. 140CPCh. 17 - Prob. 141CPCh. 17 - The overall reaction in the lead storage battery...Ch. 17 - Consider the following galvanic cell: Calculate...Ch. 17 - Prob. 144CPCh. 17 - A galvanic cell is based on the following...Ch. 17 - Prob. 146CPCh. 17 - The measurement of pH using a glass electrode...Ch. 17 - Prob. 148CPCh. 17 - A galvanic cell is based on the following...Ch. 17 - Prob. 150CPCh. 17 - Prob. 151CPCh. 17 - Prob. 152CPCh. 17 - Consider the following galvanic cell: A 15 0-mole...Ch. 17 - When copper reacts with nitric acid, a mixture of...Ch. 17 - The following standard reduction potentials have...Ch. 17 - An electrochemical cell is set up using the...Ch. 17 - Three electrochemical cells were connected in...Ch. 17 - A silver concentration cell is set up at 25C as...Ch. 17 - A galvanic cell is based on the following...Ch. 17 - Prob. 160MP
Additional Science Textbook Solutions
Find more solutions based on key concepts
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY