
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 17, Problem 17.43P
Interpretation Introduction
Interpretation:
Form the given molecules, we need to find out which compound undergoes Keto-enol tautomers.
Concept Introduction:
A carbonyl compound that has a-hydrogen on a a-carbon changes its form to enol through resonance. Both the form stays in equilibrium.
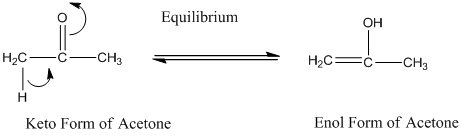
a-hydrogen is the hydrogen which attached to a a-carbon for a carbonyl compound. a-carbon is carbon adjacent to the carbonyl carbon. If a compound don’t have any a hydrogen is does not undergo Keto-Enol tautomerism.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Nitration of Methyl Benzoate:
1. Predict the major product for the reaction below AND provide a mechanism. Include ALL resonance structures for the
intermediate.
C(CH3)3
NO₂*
?
2. Assuming the stoichiometry is 1:1 for the reaction above, what volume of concentrated nitric acid would be required to
mononitrate 0.50 grams of the compound above?
What product(s) might you expect if you nitrated phenol instead of methyl benzoate? Explain your reasoning.
What signals appeared/disappeared/shifted that indicate that you have your intended product and not starting material? What
other impurities are present in your product and how do you know?
Sodium Borohydride Reduction (continued on the next page):
1. Draw the product of each of the reactions below and give the formula mass to the nearest whole number.
?
(1) NaBH
(2) acid
(1) NaBD4
(2) acid
?
2.
In mass spectra, alcohols typically break as shown in equation 8 in chapter 11 (refer to your lab manual). The larger group is
generally lost and this gives rise to the base peak in the mass spectrum. For the products of each of the reactions in question
# 1, draw the ion corresponding to the base peak for that product and give its mass to charge ratio (m/z).
3. Given the reaction below, calculate how many mg of 1-phenyl-1-butanol that can be produced using 31 mg NaBH4 and an
excess of butyrophenone.
4.
+ NaBH4
OH
(after workup with dilute
HCI)
What signals appeared/disappeared/shifted that indicate that you have your intended product and not starting material? What
other impurities are present in your product and how do you know?
Aspirin from Wintergreen:
1. In isolating the salicylic acid, why is it important to press out as much of the water as possible?
Write a step-by-step mechanism for the esterification of salicylic acid with acetic anhydride catalyzed by concentrated H₂SO4.
3. Calculate the exact monoisotopic mass of aspirin showing your work.
What signals appeared/disappeared/shifted that indicate that you have your intended product and not starting material? What
other impurities are present in your product and how do you know?
Chapter 17 Solutions
Introduction to General, Organic and Biochemistry
Ch. 17.2 - Problem 17-1 Wrtie the IUPAC name for each...Ch. 17.2 - Prob. 17.2PCh. 17.2 - Prob. 17.3PCh. 17.4 - Prob. 17.4PCh. 17.4 - Prob. 17.5PCh. 17.4 - Problem 17-6 Show the reaction of benzaldehyde...Ch. 17.4 - Problem 17-7 Identify all hemiacetals and acetals...Ch. 17.5 - Prob. 17.8PCh. 17 - 17-9 Answer true or false. (a) The one aldehyde...Ch. 17 - Prob. 17.10P
Ch. 17 - 17-11 What is the difference in structure between...Ch. 17 - 17-12 Is it possible for the carbon atom of a...Ch. 17 - 17-13 Which compounds contain carbonyl groups?Ch. 17 - 17-14 Following are structural formulas for two...Ch. 17 - 17-15 Draw structural formulas for the four...Ch. 17 - Prob. 17.16PCh. 17 - Prob. 17.17PCh. 17 - 17-18 Draw structural formulas for these ketones....Ch. 17 - 17-19 Write the JUPAC names for these compounds.Ch. 17 - Prob. 17.20PCh. 17 - 17-2 1 Explain why each name is incorrect. Write...Ch. 17 - Prob. 17.22PCh. 17 - Prob. 17.23PCh. 17 - 17-24 In each pair of compounds, select the one...Ch. 17 - Prob. 17.25PCh. 17 - 17-26 Account for the fact that acetone has a...Ch. 17 - 17-27 Pentane, 1-butanol, and butanal all have...Ch. 17 - 17-28 Show how acetaldehyde can form hydrogen...Ch. 17 - 17-29 Why can’t two molecules of acetone form a...Ch. 17 - 17-30 Answer true or false. (a) The reduction of...Ch. 17 - 17-3 1 Draw a structural formula for the principal...Ch. 17 - Prob. 17.32PCh. 17 - 17-33 What simple chemical test could you use to...Ch. 17 - 17-34 Explain why liquid aldehydes are often...Ch. 17 - 17-35 Suppose that you take a bottle of...Ch. 17 - 17-36 Explain why the reduction of an aldehyde...Ch. 17 - Prob. 17.37PCh. 17 - Prob. 17.38PCh. 17 - Prob. 17.39PCh. 17 - Prob. 17.40PCh. 17 - Prob. 17.41PCh. 17 - Prob. 17.42PCh. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Prob. 17.46PCh. 17 - 17-47 What is the characteristic structural...Ch. 17 - Prob. 17.48PCh. 17 - Prob. 17.49PCh. 17 - Prob. 17.50PCh. 17 - Prob. 17.51PCh. 17 - Prob. 17.52PCh. 17 - Prob. 17.53PCh. 17 - 17-54 Following is the structure of...Ch. 17 - Prob. 17.55PCh. 17 - Prob. 17.56PCh. 17 - Prob. 17.57PCh. 17 - Prob. 17.58PCh. 17 - Prob. 17.59PCh. 17 - 17-60 1-Propanol can be prepared by the reduction...Ch. 17 - Prob. 17.61PCh. 17 - 17-62 Show how to bring about these conversions....Ch. 17 - Prob. 17.63PCh. 17 - Prob. 17.64PCh. 17 - Prob. 17.65PCh. 17 - Prob. 17.66PCh. 17 - 17-67 Draw structural formulas for these...Ch. 17 - Prob. 17.68PCh. 17 - 17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C)...Ch. 17 - 17-70 What simple chemical test could you use to...Ch. 17 - Prob. 17.71PCh. 17 - 17-72 The following molecule is an enediol; each...Ch. 17 - 17-73 Alcohols can be prepared by the...Ch. 17 - 17-74 Glucose, C6H12O6, contains an aldehyde group...Ch. 17 - Prob. 17.75PCh. 17 - Prob. 17.76PCh. 17 - Prob. 17.77PCh. 17 - 17-78 Complete the following equation for these...Ch. 17 - 17-79 Write an equation for each conversion. (a)...
Knowledge Booster
Similar questions
- Synthesis of Ibuprofen-Part 1: 1. What characteristic absorption band changes would you expect in the IR spectrum on going from p-isobutylacetophenone to 1-(4-isobutylphenyl)-ethanol and then to 1-(4-isobutylphenyl)-1-choroethane as you did in the experiment today? Give approximate wavenumbers associated with each functional group change. Given that the mechanism of the chlorination reaction today involves formation of a benzylic carbocation, explain why the following rearranged product is not formed. محرم محمد 3. Why do we use dilute HCl for the first step of the reaction today and concentrated HCI for the second step? What signals appeared/disappeared/shifted that indicate that you have your intended product and not starting material? What other impurities are present in your product and how do you know?arrow_forwardAssign only the C NMRarrow_forwardDraw out the SALCs of wach orbital in a AlCl3 molecule.arrow_forward
- Which of the following is 3-ethyl-2-methylpentane? хarrow_forwardCan you please help me with this problem and explain it step by step? I'm so confused about itarrow_forward2. Identify the reagents you would need to achieve the following. You may need to consider using a protecting group. HO 1. 2. 3. 4. 5. OH Br HOarrow_forward
- BeF2 exists as a linear molecule. Which kind of hybrid orbitals does Be use in this compound? Use Orbital Diagrams to show how the orbitals are formed. (6)arrow_forwardPlease answer the questions and provide detailed explanations as well as a drawing to show the signals in the molecule.arrow_forwardPropose an efficient synthesis for the following transformation: EN The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. t-BuOK B. Na2Cr2O7, H2SO4, H2O C. NBS, heat F. NaCN D. MeOH E. NaOH G. MeONa H. H2O I. 1) O3; 2) DMSarrow_forward
- Stereochemistry Identifying the enantiomer of a simple organic molecule 1/5 Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of t above box under the table. Br ま HO H 0 Molecule 1 Molecule 2 Molecule 3 OH H Br H H" Br OH Br Molecule 4 Br H OH + + OH Molecule 5 Br H OH none of the above Molecule 6 Br H... OHarrow_forwardPlease answer the questions and provide detailed explanations.arrow_forwardQuestion 16 0/1 pts Choose the correct option for the following cycloaddition reaction. C CF3 CF3 CF3 CF3 The reaction is suprafacial/surafacial and forbidden The reaction is antarafacial/antarafacial and forbidden The reaction is antarafacial/antarafacial and allowed The reaction is suprafacial/surafacial and allowedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning