
Concept explainers
(a)
Interpretation:
The product formed when Propanal reacts with Methanol.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an
For an Example:
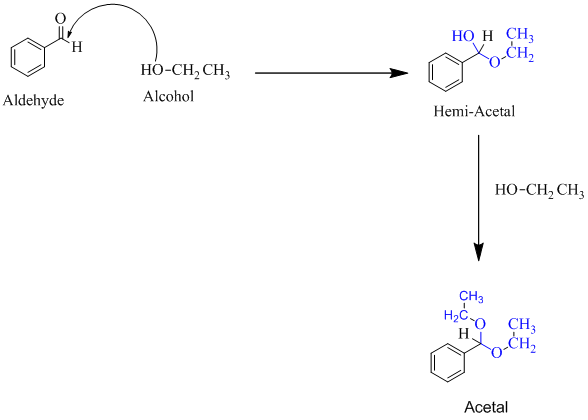
(b)
Interpretation:
The product formed when Cyclopentanone reacts with Methanol.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone.
For an Example:
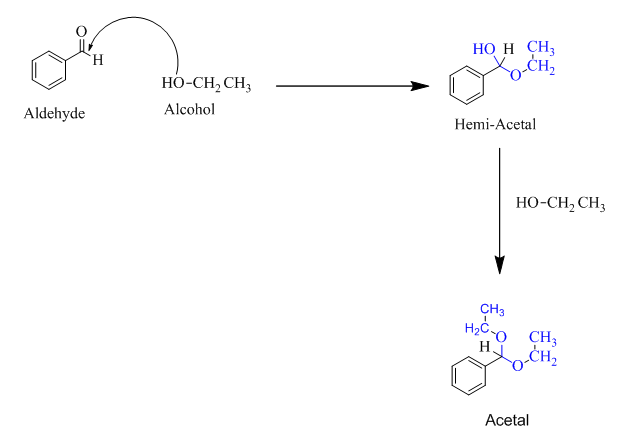
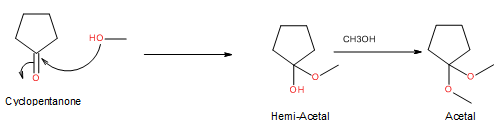
Trending nowThis is a popular solution!

Chapter 17 Solutions
Introduction to General, Organic and Biochemistry
- Name the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forward
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
