
Concept explainers
a)
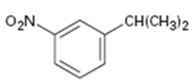
Interpretation:
The
Concept introduction:
The benzene ring is inert to even strong oxidizing agents like KMnO4 and Na2Cr2O7. However the alkyl side chains with benzylic hydrogen atoms are readily oxidized by these reagents into carboxyl groups. Compounds having no benzylic hydrogen atoms in the side chain are not oxidized.
To give:
The aromatic substance obtained as the product, when m-nitroisopropyl benzene is oxidized with KMnO4.
b)
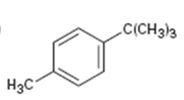
Interpretation:
The aromatic substance obtained as the product, when p-tert-butyltoluene is oxidized with KMnO4, is to be given.
Concept introduction:
The benzene ring is inert to even strong oxidizing agents like KMnO4 and Na2Cr2O7. However the alkyl side chains with benzylic hydrogen atoms are readily oxidized by these reagents into carboxyl groups. Compounds having no benzylic hydrogen atoms in the side chain are not oxidized.
To explain:
The aromatic substance obtained as the product when p-tert-butyl toluene is oxidized with KMnO4.
Trending nowThis is a popular solution!

Chapter 16 Solutions
ORGANIC CHEMISTRY W/OWL
- Can u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forwardUse IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forward
- Q5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forwardFind chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forward
- Jaslev Propose a synthesis of the following starting from benzene and any other reagents and chemicals. No mechanisms are required. Indicate the condition for each step plus the major product for each step. More than two steps are required. Step 1 Step 2 مہد Brarrow_forwardPart C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forwardPart D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forward
- Part B: The line formula for a branched alkane is shown below. a. What is the molecular formula of this compound? Number of C. Number of H b. How many carbon atoms are in the longest chain? c. How many alkyl substituents are attached to this chain?arrow_forward24. What is the major product for the following reaction? Mg J. H.C CH H,C- Then H₂O OH Br C HO E HO H.C CH H.C- CH₂ CH₂ All of these are possiblearrow_forwardstructures. Explain why the major product(s) are formed over the minor product(s) using the Draw the major and product and the complete mechanism for all products with all resonance mechanism/resonance structures of the major and minor products in your explanation. HONO2 H2SO4arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

