
a)
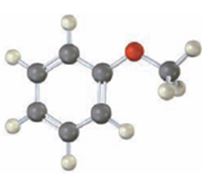
Interpretation:
Draw the products obtained when the compound shown (methoxybenzene) reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Concept introduction:
Aromatic rings can be brominated using Br2 in the presence of FeBr3. The electrophile is Br+. Friedal-Crafts acylation reaction occurs when an
To draw:
The products obtained when the compound shown (methoxybenzene) reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Answer to Problem 24VC
(i) The products obtained when the compound shown (methoxybenzene) reacts with Br2/FeBr3 are p-bromomethoxybenzene and o-bromomethoxybenzene.
(ii) The products obtained when the compound shown (methoxybenzene) reacts with CH3COCl/AlCl3 are p-acetylmethoxybenzene and o-acetylmethoxybenzene.
Explanation of Solution
The methoxy group attached to the benzene ring is an ortho & para directing group. When methoxybenzene is brominated and alkylated it directs the incoming electrophiles, Br+ and CH3CO+ to ortho & para positions.
(i) The products obtained when the compound shown (methoxybenzene) reacts with Br2/FeBr3 are p-bromomethoxybenzene and o-bromomethoxybenzene.

(ii) The products obtained when the compound shown (methoxybenzene) reacts with CH3COCl/AlCl3 are p-acetylmethoxybenzene and o-acetylmethoxybenzene.

b)
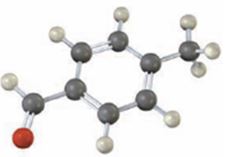
Interpretation:
Draw the structures of the products obtained when the compound shown, p-methylbenzaldehyde reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Concept introduction:
Electrophilic substitution of disubstituted benzenes follows three simple rules. (i) If the directing influence of both the substituents reinforce each other, a single product results. (ii) If the directing influences of both the substituent groups oppose each other, the most powerful activating group among them has the dominant influence but usually a mixture of products results. (iii) In meta disubstituted compounds, further substitution in between the groups occurs only rarely, due to steric reasons.
To show:
The major product(s) produced when p-methylbenzaldehyde reacts with (i) Br2, FeBr3 and (2) CH3COCl, AlCl3.
Answer to Problem 24VC
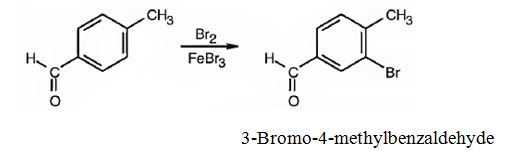
(i) The major product produced when p-methylbenzaldehyde reacts with Br2/FeBr3 is 3-bromo-4-methylbenzaldehyde.
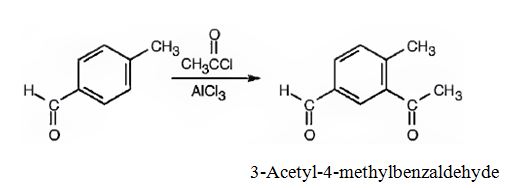
(ii) The major product produced when p-methylbenzaldehyde reacts with CH3COCl/AlCl3 is 3-acetyl-4-methylbenzaldehyde.
Explanation of Solution
The
(i) The major product produced when p-methylbenzaldehyde reacts with Br2/FeBr3 is 3-bromo-4-methylbenzaldehyde.

(ii)The major product produced when p-methylbenzaldehyde reacts with CH3COCl/AlCl3 is 3-acetyl-4-methylbenzaldehyde.

Want to see more full solutions like this?
Chapter 16 Solutions
ORGANIC CHEMISTRY W/OWL
- Which of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forwardCan I get help drawing my arrows #2arrow_forward
- Can I get some help with my arrows? I have included what the final outcome needs to look like. #3arrow_forwardPlease explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


