
Rationalize the difference in boiling points for each ofthe following pairs of substances:
a. n-pentane
b. HF 20°C
HCl -85°C
c. HCl-85°C
LiCl 1360°C
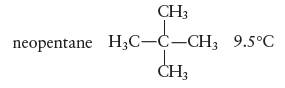
d. n-pentane
n-hexane
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
EBK CHEMICAL PRINCIPLES
- What feature characterizes the dynamic equilibrium between a liquid and its vapor in a closed container?arrow_forwardWhat phase changes will take place when water is subjected to varying pressure at a constant temperature of 0.005 C? At 40 C? At 40 C?arrow_forward8.48 Why must the vapor pressure of a substance be measured only after dynamic equilibrium is established?arrow_forward
- Consider the following data for xenon: Triple point: 121C, 280 torr Normal melting point: 112C Normal boiling point: 107C Which is more dense, Xe(s) or Xe(l)? How do the melting point and boiling point of xenon depend on pressure?arrow_forwardou seal a container half-filled with water. Which best describes what occurs in the container? Water evaporates until the air becomes saturated with water vapor; at this point, no more water evaporates. Water evaporates until the air becomes overly saturated (supersaturated) with water, and most of this water recondenses; this cycle continues until a certain amount of water vapor is present, and then the cycle ceases. The water does not evaporate because the container sealed. Water evaporates, and thou water evaporates and recondenses simultaneously and continuously. The water evaporates until it is eventually all in vapor form. stify your choice, and for choices you did not pick, explain what is wrong with them.arrow_forwardLiquid methanol, CH3OH, is placed in a glass tube. Is the meniscus of the liquid concave or convex? Explain briefly.arrow_forward
- How does the boiling of a liquid differ from its evaporation?arrow_forwardThe halogens form a series of compounds with each other, which are called interhalogens. Examples are bromine chloride (BrCl), iodine bromide (IBr), bromine fluoride (BrF), and chlorine fluoride (ClF). Which compound is expected to have the highest boiling point at any given pressure? Explain.arrow_forwardDescribe the behavior of a liquid and its vapor in a closed vessel as the temperature increases.arrow_forward
- For each of the following pairs, choose the member with the lower boiling point. Explain your reason in each case. (a) NaCl or PCl3 (b) NH3 or AsH3 (c) C3H7OH or C2H5OCH3 (d) HI(g) or HCl(g)arrow_forwardCarbon disulfide, CS2 is a volatile, flammable liquid. It has a vapor pressure of 400.0 mmHg at 28.0C and 760.0 mmHg at 46.5C. What is the heat of vaporization of this substance?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





