
(a)
Interpretation: The formula of the four superconductors needs to be determined.
Concept Introduction: The superconductor is a substance which is capable of superconducting at very low temperature values.
(a)
Explanation of Solution
The given structures are as follows:
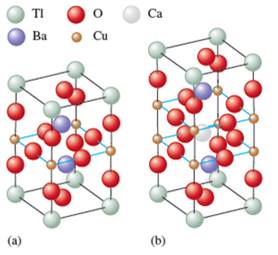
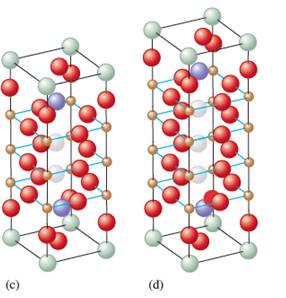
By considering the color code for spheres, the number of atoms of each element can be counted.
It can be observed that in molecule (a) there is 1 Tl, 2 Ba, 1 Cu and 5 O atoms in 1unit. Thus, the formula of the molecule will be:
Similarly, the atoms in molecule (b) are 1 Tl, 2 Ba, 1 Ca, 1 Cu and 7 O in 1unit. Thus, the formula will be:
The structure (c) contains 1 Tl, 2 Ba, 2 Ca, 3 Cu and 9 O atoms in 1unit. The formula will be:
Similarly, the structure (d) has 1 Tl, 2 Ba, 2 Ca, 4 Cu and 11 O atoms in 1unit thus, the molecular formula will be:
(b)
Interpretation: The four structures needs to be ordered from lowest to highest superconducting temperature.
Concept introduction: The number of sheets in the unit cell is directly proportional to the temperature for the superconductivity.
(b)
Explanation of Solution
If the number of sheets in each unit cell increases, the temperature for the superconductivity increases. The number of sheets in the structure (a) is less then (b) which is further less than (c) and (d). Thus, the increasing order of the superconductivity for 4 structures will be as follows:
(c)
Interpretation: The oxidation state needs to be assigned to Cu in each mixture. It is assumed that the oxidation state of Tl is +3. Also, the oxidation state of Ca, Ba, O is +2, +2 and -2 respectively.
Concept Introduction: The oxidation state of any atom in the molecule is equal to the charge on it. It can be calculated if oxidation state of all the other atoms is given.
For example, the molecule H2O2 is neutral. The oxidation state of H is +1 (general) thus, the oxidation state of O can be calculated as follows:
Thus, the oxidation state of O in H2O2 is -1.
(c)
Explanation of Solution
The oxidation state of Cu in structure can be calculated by taking the overall charge on the molecule equal to zero.
The oxidation state of Tl, Ca, Ba and O is assumed +3, +2, +2 and -2 respectively.
For
Thus, the oxidation state of Cu in molecule (a) is +3.
For
There are 2 Cu atoms, thus, there is a mixed oxidation state one is
For
Thus, copper has mixed oxidation state that is 1
For
There will be one
(d)
Interpretation: The reason for the copper to display a mixture of oxidation states in the superconductor needs to be explained.
Concept Introduction: The superconductor is a substance which is capable of superconducting at very low temperature values.
(d)
Explanation of Solution
There are variable oxidation states of copper due to varying the numbers of Ca, Cu and O atoms in each unit cell. The different oxidation states of Cu are calculated for molecules (b), (c) and (d). In the other superconductor in exercise 79, in YBa2Cu3O7, there is variable oxidation state of copper by omitting oxygen atom at various sites in the lattice.
Want to see more full solutions like this?
Chapter 16 Solutions
EBK CHEMICAL PRINCIPLES
- Predict the major products of this organic reaction: H OH 1. LiAlH4 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. G C टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forward
- Predict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forward
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forwardgive example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward
- 2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





