
Concept explainers
(a)
Interpretation:
To check whether the statement, “Aqueous solutions of
Concept Introduction:
Lewis acids are the ones which can accept a pair of electrons. Lewis bases are the ones which donate a pair of electrons. So, a substance which can donate a pair of electrons can be termed as a base.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

From the reaction, it is clear that the nitrogen donates a pair of electrons to hydrogen thus, making a salt. Thus, aqueous solutions of amines are basic in nature.
Hence, the statement is True.
(b)
Interpretation:
To check whether the statement, “
Concept Introduction:
Lewis acids are the ones which can accept a pair of electrons. Lewis bases are the ones which donate a pair of electrons. So, a substance which can donate a pair of electrons can be termed as a base.
Explanation of Solution
Aniline is the benzene ring that is attached to amine group. The lone pair of electrons present on the nitrogen atom is in conjugation with the aromatic ring. Thus, the lone pair of electrons is less available to donate to a proton. Hence, aniline is less basic in nature in comparison with methyl amine.
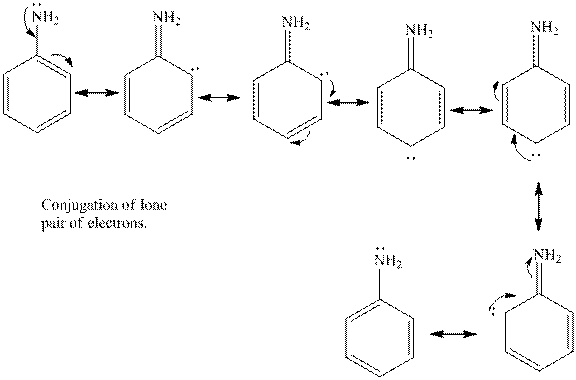
The cyclohexylamine is the one where the cyclohexyl ring is attached to the amine group. Hence, it is similar to an alkyl group attached to amine group. The alkyl groups are electron donating in nature due to inductive effect. Hence, the basicity of the amine nitrogen increases due to the attached cyclohexyl ring. Hence, the compound is more basic.
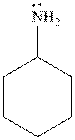
Thus, cyclohexylamine is more basic than aniline. Hence, aromatic amines are weaker bases than aliphatic amines.
The given statement is True.
(c)
Interpretation:
To check whether the statement, “Aliphatic amines are stronger bases than inorganic bases such as NaOH and KOH” is true or false.
Concept Introduction:
Generally, the strength of a base is compared on the basis that how readily the base produces the hydroxide ion. This can be either by releasing the hydroxide ion which they readily have and may be because they take hydrogen ions from the water and thus they can produce hydroxide ion.
Explanation of Solution
Generally, the strength of a base is compared on the basis that how readily the base produces the hydroxide ion. This can be either by releasing the hydroxide ion which they readily have and may be because they take hydrogen ions from the water and thus they can produce hydroxide ion.
NaOH and KOH are the acids which readily give hydroxide ions when added to water. They dissociate completely. The reaction is not reversible and hence, they are strong acids.
Aliphatic amines with general formula

Hence, the statement is False.
(d)
Interpretation:
To check whether the statement, “Water insoluble amines react with strong aqueous acids such as HCl to form water soluble salts” is true or false.
Concept Introduction:
Water has a property to ionize salts. This property helps in dissolution of salts in water.
Explanation of Solution
Water is an universal solvent. Its polarity and many other aspects make it an unique solvent. It also has the property to ionize. This property makes salts to be easily dissolved in water. For this reason, amines which are usually insoluble in water are made soluble by converting into hydrochloride salts.
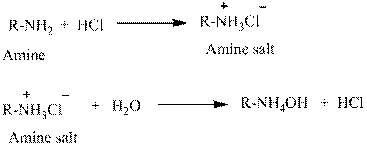
(e)
Interpretation:
To check whether the statement, “If the pH of an aqueous solution of a primary aliphatic amine is adjusted to pH 2.0 by the addition of concentrated HCl, the amine will be present in solution almost entirely as its conjugate acid” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. Given that pH is 2.0. That means, it is on the acidic side. As the acidic environment is present, the species that will present is the amine salt.
Hence, the statement is True.
(f)
Interpretation:
To check whether the statement, “If the pH of an aqueous solution of a primary aliphatic amine is adjusted to pH 10.0 by the addition of NaOH, the amine will be present in solution almost entirely as the free base” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. Given that pH is 10.0 by addition of NaOH. That means, the pH is on the basic side which is because of the added NaOH. As the basic environment is present, the species that will present is the basic amine.
Hence, the statement is True.
(g)
Interpretation:
To check whether the statement, “For a primary amine, the concentrations of salt and basic amine will be equal when the pH of the solution is equal to the pKb of the amine” is true or false.
Concept Introduction:
The nature of the compound depends on its similarity of the chemical environment. The balance of a reaction is according to Le Chatlier’s principle.
Explanation of Solution
The general formula of amines is
Let us consider its reaction with water.

The reactant amine is basic in nature, and the conjugate acid is the product. At certain pH such as pKb the system is at equilibrium. pKb is the equilibrium dissociation constant pH. That means, the pH is so supporting that the forward and reverse reactions are at equal rate. That means, there will be forward and reverse reactions but that is not noticeable because they are at equal rate.
Hence, at this pH, the concentrations of salt and the basic salt will be equal. Hence, the statement is True.
Want to see more full solutions like this?
Chapter 16 Solutions
Introduction to General, Organic and Biochemistry
- Can anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forwardDraw the Fischer projection of D-fructose. Click and drag to start drawing a structure. Skip Part Check AP 14 tv SC F1 F2 80 F3 a F4 ! 2 # 3 CF F5 75 Ax MacBook Air 894 $ 5olo % Λ 6 > W F6 K F7 &arrow_forward
- Consider this step in a radical reaction: Y What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ionization propagation initialization passivation none of the abovearrow_forward22.16 The following groups are ortho-para directors. (a) -C=CH₂ H (d) -Br (b) -NH2 (c) -OCHS Draw a contributing structure for the resonance-stabilized cation formed during elec- trophilic aromatic substitution that shows the role of each group in stabilizing the intermediate by further delocalizing its positive charge. 22.17 Predict the major product or products from treatment of each compound with Cl₁/FeCl₂- OH (b) NO2 CHO 22.18 How do you account for the fact that phenyl acetate is less reactive toward electro- philic aromatic substitution than anisole? Phenyl acetate Anisole CH (d)arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forward
- Help me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forwardIs an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forward
- Help me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forwardV Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





