
Concept explainers
(a)
Interpretation: To determine whether glutamate and aspartate could function as the two reactants in a transamination reaction or not.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
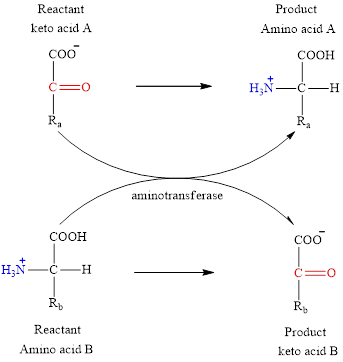
The general structure of an amino acid is:
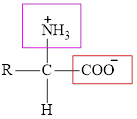
Here,
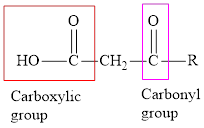
An acid containing both carbonyl and carboxyl
(a)
Answer to Problem 15.34EP
No, glutamate and aspartate cannot function as the reactants in a transamination reaction.
Explanation of Solution
Glutamate is an amino acid and its structure is:
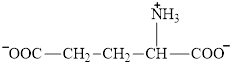
Aspartate is an amino acid and its structure is:
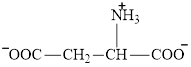
The two reactants in transamination reaction are a keto acid and an amino acid. Both glutamate and aspartate are amino acids thus they cannot function as reactants in a transamination reaction. For a transamination reaction to take place there must be one amino acid present along with a keto acid.
(b)
Interpretation: To determine whether aspartate and
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an

The general structure of an amino acid is:

Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:

(b)
Answer to Problem 15.34EP
Yes, aspartate and
Explanation of Solution
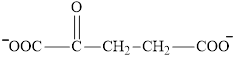
Aspartate is an amino acid and its structure is:
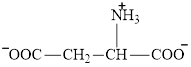
Transamination reaction involves the exchange of an amino group from an
(c)
Interpretation: To determine whether succinate and
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an

The general structure of an amino acid is:

Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:

(c)
Answer to Problem 15.34EP
No, succinate and
Explanation of Solution
Succinate is a diacid acid and its structure is:
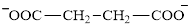
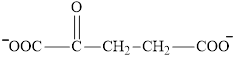
The two reactants in transamination reaction are a keto acid and an amino acid.
(d)
Interpretation: To determine whether glutarate and aspartate could function as the two reactants in a transamination reaction or not.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
The general reaction to illustrate transamination is as follows:

The general structure of an amino acid is:

Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:

(d)
Answer to Problem 15.34EP
No, glutarate and aspartate cannot function as the reactants in a transamination reaction.
Explanation of Solution
Aspartate is an amino acid and its structure is:
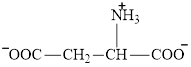
Glutarate is a diacid and its structure is:
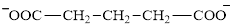
The two reactants in transamination reaction are a keto acid and an amino acid. Aspartate is an amino acid but glutarate is not a keto acid. For a transamination reaction to take place there must be one keto acid present along with an amino acid. Thus, glutarate and aspartate cannot function as the reactants in a transamination reaction.
Want to see more full solutions like this?
Chapter 15 Solutions
Organic And Biological Chemistry
- Michael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forwardRank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forward
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning





