
Concept explainers
Stock solutions of HCl with various molarities are frequentlyprepared. Complete Table 14.7 by calculatingthe volume of concentrated, or 12M, hydrochloric acidthat should be used to make 1.0 L of HCl solution witheach molarity listed.
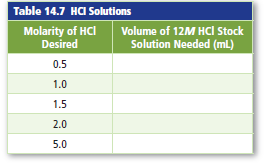
Interpretation:
Volume of the stocksolutions have to be calculated.
Concept introduction:
The equation for dilution is
Concentrated solutions of known molarities are known as stock solution.
Where,
M1 = molarity of the stock solution V1 = volume of stock solution
M2 = molarity of the diluted solution V2 = volume of diluted solution
Answer to Problem 75A
| Molarity of HCl desired | Volume of 12M HCl stock solution needed (mL) |
| 0.5 | 41.7 |
| 1.0 | 83.3 |
| 1.5 | 125 |
| 2.0 | 167 |
| 5.0 | 417 |
Explanation of Solution
Data given:
M1 = molarity of the stock solution = 12 M
M2 = molarity of the diluted solution
V2 = volume of diluted solution = 1 L
- Volume of 12M HCl stock solution needed to make 0.5 M diluted solution
- Volume of 12M HCl stock solution needed to make 1.0 M diluted solution
- Volume of 12M HCl stock solution needed to make 1.5 M diluted solution
- Volume of 12M HCl stock solution needed to make 2.0 M diluted solution
- Volume of 12M HCl stock solution needed to make 5.0 M diluted solution
Chapter 14 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Anatomy & Physiology (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology (11th Edition)
Microbiology with Diseases by Body System (5th Edition)
Applications and Investigations in Earth Science (9th Edition)
- Can someone help me whats the issue?arrow_forwarda. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression: AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T A. Calculate the value of AS for the process. B. Next, use the Gibbs-Helmholtz equation: (a(AG/T)) ΔΗ - T2 to calculate the value of AH for the process.arrow_forwardNonearrow_forward
- ASP please....arrow_forwardNonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forward
- Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





