
Concept explainers
Fifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution
(a) before any NaOH is added?
(b) at half-neutralization?
(c) at the equivalence point?
(d) when 0.10 mL less than the volume of NaOH to reach the equivalence point is added?
(e) when 0.10 mL more than the volume of NaOH to reach the equivalence point is added?
(f) Use your data to construct a plot similar to that shown in Figure 14.10 (pH versus volume NaOH added).
(a)
Interpretation:
The titration of NaOH of molarity 0.850 M occurs with nitrous acid of 50 cm3 with molarity 1.000M. The pH value of the solution is to be determined before the addition of NaOH.
Concept introduction:
For a reaction as follows:
The equilibrium constant can be calculated as follows:
The pH of the solution can be calculated as follows:
Answer to Problem 74QAP
The pH value of the solution is 1.62.
Explanation of Solution
The dissociation reaction for the nitrous acid is shown below-
Given that-
Molarity of HNO2 = 1.000M
The dissociation constant, Ka = 6.0×10-4
Consider the x M of HNO2 is ionized. Hence the ICE table is shown below-
| HNO2 | H+ | NO2 - | |
| I (mol) | 1.000 | 0 | 0 |
| C (mol) | -x | +x | +x |
| E (mol) | 1.000 -x | x | x |
Now the dissociation constant Ka is calculated as-
Given that-
[HNO2 ] = 1.000 -x
[H+] = x
[NO2 -] = x
Put the above values in equation (1),
On calculation −
x = 2.42×10-2
The concentration of [H+] = x= [NO2 -] = 2.42×10-2 at equilibrium
Now, the pH value is −
The value of pH = 1.62
(b)
Interpretation:
The pH value of the solution is to be determined at half neutralization.
Concept introduction:
The
The pH of the solution can be calculated as follows:
Answer to Problem 74QAP
The pH value of the solution is 3.22 at half neutralization.
Explanation of Solution
At half neutralization, only half acid will be ionized and the equilibrium concentration of the conjugate base of acid and acid will be equal. Therefore,
Or
And
Ka = 6.0×10-4
The value of pH for the solution = 3.22
(c)
Interpretation:
The pH value of the solution is to be determined at the equivalence point.
Concept introduction:
The molarity of solution is calculated as follows:
Here, number of moles is of solute and volume is of solution.
For a base dissociation reaction,
The base dissociation constant will be:
It is related to acid dissocation constant and ionization constant of water as follows:
The pOH and pH of the solution can be calculated as follows:
Here,
Answer to Problem 74QAP
The pH value of the solution is 8.45 at the equivalence point.
Explanation of Solution
The chemical equation for the reaction between nitrous acid and sodium hydroxide is given as-
The net-ionic equation of the above reaction is −
Number of moles of nitrous acid-
Or
Given that-
Molarity = 1.000M
Volume = 50.0cc = 0.050L
Put the above values in equation (1)
Number of moles of HNO2 = 0.0500 mol
At equivalence point-
Number of moles of NaOH added = initial number of moles of HNO2 = 0.0500 mol
Molarity of the base = 0.850 M
The volume of the base-
Put the values in the above equation-
Volume of base = 0.0588 L
Molarity of NO2 - is calculated as-
Number of moles of HNO2 = 0.0500mol = Number of moles of NO2 -
Total volume = 0.0500L +0.0588L
Put the given values in above equation-
Molarity of NO2 - = 0.459M
Consider the M of NO2 - will be ionized. Hence the ICE table is shown below-
| NO2 - | HNO2 | OH- | |
| I (mol) | 0.459 | 0 | 0 |
| C (mol) | -y | +y | +y |
| E (mol) | 0.459 -y | y | y |
Now the base dissociation constant Kb is calculated as-
Given that-
[NO2 -] = 0.459 −y
[HNO2 ] = y
[OH-] = y
Put the above values in equation (2)
On calculation −
[OH-] = y = 2.79×10-6
Now, the pH of the solution −
And,
(d)
Interpretation:
The volume less than the 0.10 mL of NaOH is added to reach the equivalence point. At this condition, the pH value of the solution is to be determined.
Concept introduction:
The molarity of solution is calculated as follows:
Here, number of moles is of solute and volume is of solution.
Now, from the Henderson −Hasselbalch equation is as follows−
Answer to Problem 74QAP
The pH value of the solution is 5.92, when the volume less than the 0.10mL of NaOH added.
Explanation of Solution
The number of moles of base-
Given that-
Volume = 58.7mL (0.1mL less than 58.8mL)
Molarity = 0.850 M
Put the above values in Equ (1)
Number of moles of base (NaOH) = 0.0499mol
Now, the number of moles of HNO2 −
Now, the number of moles of conjugate base, NO2 -
Now, from the Henderson −Hasselbalch equation −
Or,
On calculation-
(e)
Interpretation:
The volume more than the 0.10mL of NaOH is added to reach the equivalence point. At this condition, the pH value of the solution is to be determined.
Concept introduction:
The molarity of solution is calculated as follows:
Here, number of moles is of solute and volume is of solution.
The pH of the solution can be calculated as follows:
Answer to Problem 74QAP
The pH value of the solution is 10.88 when the volume more than the 0.10mL of NaOH added.
Explanation of Solution
The number of moles of base-
Given that-
Volume = 58.9mL (0.1mL greater than 58.8mL)
Molarity = 0.850 M
Put the above values in equation (1)
Number of moles of base (NaOH) = 0.050082mol
Now, the number of moles of OH- −
Now, pOH-
And
(f)
Interpretation:
The graph is to be plotted by using the calculated data between pH vs. NaOH added.
Concept introduction:
The equivalence point is defined as the point for the titration process at which addition of titrant is enough for the complete neutralization of the analyte.
Half neutralization is defined as the point at which the concentration of weak acid will become equal to its conjugate base.
Explanation of Solution
The graph plot between pH vs. NaOH added is shown below-
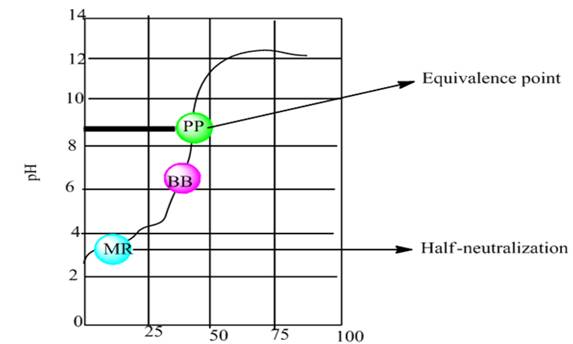
According to the graph, the half neutralization point is approximately at pH 2.8 and equivalence point is approximately at pH 8.8
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry: Principles and Reactions
- The following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forwardWhat mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward(ME EX2) Prblms Can you please explain problems to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forward
- Experiment #8 Electrical conductivity & Electrolytes. Conductivity of solutions FLINN Scientific Scale RED LED Green LED LED Conductivity 0 OFF OFF 1 Dim OFF 2 medium OFF 3 Bright Dim Low or Nowe Low Medium High 4 Very Bright Medium nd very high AA Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ SE=Strong Electrolyte, FE = Fair Electrolyte CWE = Weak Electrolyte, NE= Noni Electrolyte, #Solutions 1 0.1 M NaCl 2/1x 102 M NaCl, 3/1X103 M Nall Can Prediction M Observed Conductivity Very bright red Bright red Dim red you help me understand how I'm supposed to find the predictions of the following solutions? I know this is an Ionic compound and that the more ions in a solution means it is able to carry a charge, right? AAAA Darrow_forward(SE EX 2) Prblsm 4-7: Can you please explain problems 4-7 and color code if needed for me. (step by step) detail explanationsarrow_forward(SE EX 2) Problems 8-11, can you please explain them to me in detail and color-code anything if necessary?arrow_forward
- (ME EX2) Problems 15-16 Could you please explain problems 15 through 16 to me in detail, step by step? Thank you so much! If necessary, please color-code them for me.arrow_forward1.)show any electrophilic aromatic substitution, identify the electriphile, nucleophile and transition statearrow_forward(SE EX 2) Problems 15-16, can you please explain them to me in detail and color-code anything if necessary?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





