
Concept explainers
(a)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
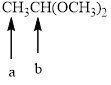
In the given structure, the indicated arrow “ a” split into doublet and “b” into quartet.
Explanation of Solution
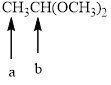
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow a and b are having nonequivalent protons. Thus methyl proton indicated by “ a” into doublet and “ b” into quartet.
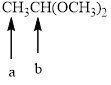
Thus in the given structure, for the indicated arrow “ a” split into doublet and “b” into quartet.
(b)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
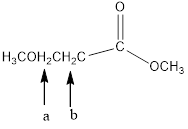
In the given structure, the indicated arrow “ a” and “b” split into doublet.
Explanation of Solution

Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow a and b are having equivalent protons. Thus proton indicated by “ a” and “b” split into doublet.

Thus in the given structure for the indicated arrow “ a” and “b” split into doublet.
(c)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
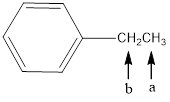
In the given structure, the indicated arrow “ a” split into triplet and “b” split into qaurtet.
Explanation of Solution

Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow a and b are having nonequivalent protons. Thus proton indicated by “ a” split into triplet and “b” split into qaurtet.

Thus in the given structure for the indicated arrow “ a” split into triplet and “b” split into qaurtet.
(d)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
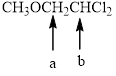
In the given structure, the indicated arrow “ a” split into doublet and “b” split into triplet.
Explanation of Solution
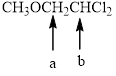
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow a and b are having nonequivalent protons. Thus proton indicated by “ a” split into doublet and “b” split into triplet.
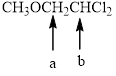
Thus in the given structure for the indicated arrow “ a” split into doublet and “b” split into triplet.
(e)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
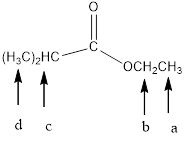
In the given structure, the indicated arrow “ a” split into triplet, “b” split into quartet,
“c” split into septet and “d” split into doublet.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow a, b, c and d are having nonequivalent protons. Thus proton indicated by “ a” split into triplet, “b” split into quartet, “c” split into septet and “d” split into doublet.


Thus in the given structure for the indicated arrow “ a” split into triplet, “b” split into quartet,
“c” split into septet and “d” split into doublet.
(f)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
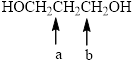
In the given structure, the indicated arrow “ a” split into quintet and “b” split into triplet.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow a and b are having nonequivalent protons. Thus proton indicated by “a” split into quintet and “b” split into triplet.
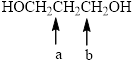
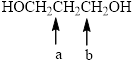
Thus in the given structure for the indicated “ a” split into quintet and “b” split into triplet.
(g)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
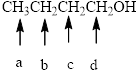
In the given structure, the indicated arrow “ a” split into triplet, “b” split into multiplet i.e it observe 5 peaks (12 peaks maximum), “c” split into multiplet i.e it observe 6 peaks (9 peaks maximum) and “d” split into triplet.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow protons are having nonequivalent protons. Thus proton indicated by “ a” split into triplet, “b” split into multiplet i.e it observe 5 peaks (12 peaks maximum), “c” split into multiplet i.e it observe 6 peaks (9 peaks maximum) and “d” split into triplet.
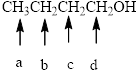
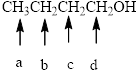
Thus in the given structure for the indicated “ a” split into triplet, “b” split into multiplet i.e it observe 5 peaks (12 peaks maximum), “c” split into multiplet i.e it observe 6 peaks (9 peaks maximum) and “d” split into triplet.
(h)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
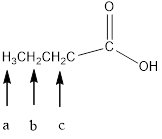
In the given structure, the indicated arrow “a” split into triplet, “b” split into multiplet i.e., it observe 6 peaks and “c” split into triplet.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow protons are having nonequivalent protons. Thus proton indicated by “a” split into triplet, “b” split into multiplet i.e., it observe 6 peaks and “c” split into triplet.

Thus in the given structure for the indicated “a” split into triplet, “b” split into multiplet i.e., it observe 6 peaks and “c” split into triplet.

(i)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
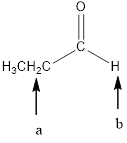
In the given structure, the indicated arrow “a” split into 8 peak (maximum) but observed singlet, “b” split into triplet.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow protons are having nonequivalent protons. Thus proton indicated by “a” split into 8 peak (maximum) but observed singlet, “b” split into triplet.

Thus in the given structure for the indicated “a” split into 8 peak (maximum) but observed singlet, “b” split into triplet.

(j)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
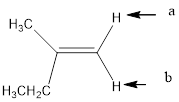
In the given structure, the indicated arrow “a” and “b” split into doublet.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow protons are having equivalent protons lie on the same carbon. Thus proton indicated by “a” and “b” split into doublet.
Thus in the given structure for the indicated “a” and “b” split into doublet.

(k)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
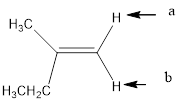
In the given structure, the indicated arrow “a” and “b” split into doublet.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow protons are having equivalent protons lie on the same carbon. Thus proton indicated by “a” and “b” split into doublet.
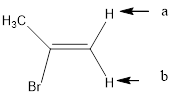
Thus in the given structure for the indicated “a” and “b” split into doublet.

(l)
Interpretation:
To determine how many peaks will the signal for the given structure of the indicated protons split.
Concept introduction:
NMR is the spectroscopic technique to observe the local magnetic fields around the atomic nuclei. The relative intensity of 1H NMR signals provide the information about the compound structure. The area under the NMR signal is proportional to the number of absorbing protons.
The height of each step is proportional to the area under the peak, which is in turn proportional to the number of absorbing protons.
Answer to Problem 14.44P
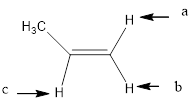
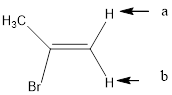
In the given structure, the indicated arrow “a” and “b” split into doublet of doublet and “c” split into maximum of 16 peaks.
Explanation of Solution
Splitting pattern determines the kind of proton of proton in the structure. First determine if two protons are equivalent or different. Only nonequivalent protons split each other and also determine if two nonequivalent protons are close enough to split each other's signals. Splitting is observed only for nonequivalent protons on the same carbon or adjacent carbons.
In the given structure the indicated arrow protons are having non-equivalent protons lie on the same carbon. Thus proton indicated by “a” and “b” split into doublet of doublet and “c” split into maximum of 16 peaks.

Thus in the given structure for the indicated “a” and “b” split into doublet of doublet and “c” split into maximum of 16 peaks.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardUsing what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forward
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardIndicate whether the copper(II) acetate dimer, in its dihydrated form with the formula [(CH3COO)2Cu]2·2H2O, is a metal cluster, a cage compound, or neither.arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
