
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.30P
Identify the carbon atoms that give rise to each NMR signal.
a. 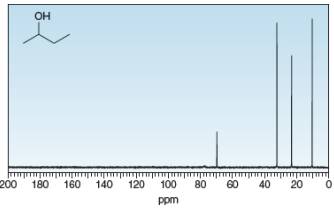
b. 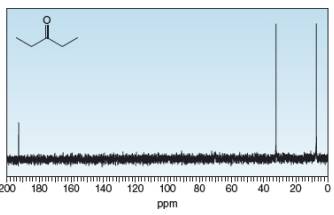
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sketch the intermediates for A,B,C & D.
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
O
? A
. If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
. If your answer is no, check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a structure.
ㅇ
80
F5
F6
A
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente
FIG
In methyl orange preparation, if the reaction started with 0.5 mole of sulfanilic acid to form the diazonium salt of this compound and then it converted to methyl orange [0.2 mole]. If the efficiency of the second step was 50%, Calculate: A. Equation(s) of Methyl Orange synthesis: Diazotization and coupling reactions. B. How much diazonium salt was formed in this reaction? C. The efficiency percentage of the diazotization reaction D. Efficiency percentage of the whole reaction.
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 14 - Prob. 14.2PCh. 14 - How many 1H NMR signals does each compound show?Ch. 14 - How many 1H NMR signals does each...Ch. 14 - Label the protons in each highlighted CH2 group as...Ch. 14 - How many 1H NMR signals would you expect for each...Ch. 14 - Prob. 14.7PCh. 14 - For each compound, first label each different type...Ch. 14 - Prob. 14.9PCh. 14 - Which compound give a 1H NMR spectrum with two...
Ch. 14 - Prob. 14.11PCh. 14 - Prob. 14.12PCh. 14 - Prob. 14.13PCh. 14 - For each compound give the number of 1H NMR...Ch. 14 - Prob. 14.15PCh. 14 - Prob. 14.16PCh. 14 - Describe the 1H NMR spectrum of each compound....Ch. 14 - Draw a splitting diagram for Hb in...Ch. 14 - Problem 14.20 Identify A and B, isomers of...Ch. 14 - Problem 14.21 How many signals are present in the ...Ch. 14 - What protons in alcohol A give rise to each signal...Ch. 14 - How many peaks are observed in the NMR signal for...Ch. 14 - Propose a structure for a compound of molecular...Ch. 14 - Problem 14.25 Propose a structure for a compound...Ch. 14 - Problem 14.26. Identify products A and B from the...Ch. 14 - How many lines are observed in the 13C NMR...Ch. 14 - Problem 14.28 Draw all constitutional isomers of...Ch. 14 - Esters of chrysanthemic acid are naturally...Ch. 14 - Prob. 14.29PCh. 14 - Problem 14.31 Identify the carbon atoms that give...Ch. 14 - Problem 14.32 A compound of molecular formula ...Ch. 14 - Problem 14.33 Draw the structure of a compound of...Ch. 14 - 14.34 (a) How many NMR signals does each of the...Ch. 14 - 14.35 (a) How many NMR signals does each compound...Ch. 14 - How many different types of protons are present in...Ch. 14 - How many 1H NMR signals does each compound give?...Ch. 14 - 14.37 How many NMR signals does each natural...Ch. 14 - Prob. 14.38PCh. 14 - Acetone exhibits a singlet in its 1H NMR spectrum...Ch. 14 - Prob. 14.40PCh. 14 - Prob. 14.41PCh. 14 - How could you use chemical shift and integration...Ch. 14 - Which compounds give one singlet in the 1H NMR...Ch. 14 - Prob. 14.44PCh. 14 - 14.43 How can you use NMR spectroscopy to...Ch. 14 - Prob. 14.46PCh. 14 - Prob. 14.47PCh. 14 - Which compounds in Problem 14.43 give one signal...Ch. 14 - Prob. 14.49PCh. 14 - How many 13C NMR signals does each compound...Ch. 14 - Rank the highlighted carbon atoms in each compound...Ch. 14 - 14.50 Identify the carbon atoms that give rise to...Ch. 14 - 14.51 a. How many signals does dimethyl...Ch. 14 - 14.53 Propose a structure consistent with each set...Ch. 14 - 14.54 Identify the structures of isomers A and B...Ch. 14 - 14.55 Reaction of with affords compound W,...Ch. 14 - 14.56 Treatment of with , followed by aqueous
...Ch. 14 - 14.57 Compound C has a molecular ion in its mass...Ch. 14 - 14.58 As we will learn in Chapter 20, reaction of ...Ch. 14 - 14.59 Identify the structures of isomers E and F...Ch. 14 - 14.59 Identify the structures of isomers H and I...Ch. 14 - 14.61 Propose a structure consistent with each set...Ch. 14 - 14.62 Reaction of with , followed by treatment...Ch. 14 - Reaction of aldehyde D with amino alcohol E in the...Ch. 14 - 14.64 Propose a structure consistent with each set...Ch. 14 - 14.65 In the presence of a small amount of acid, a...Ch. 14 - 14.66 Treatment of with affords two products (M...Ch. 14 - 14.67 Compound O has molecular formula and shows...Ch. 14 - 14.68 Compound P has molecular formula . Deduce...Ch. 14 - 14.69 Treatment of with strong base followed by ...Ch. 14 - Low molecular weight esters RCO2R often have...Ch. 14 - 14.70 When -bromo--dimethylbutane is treated with...Ch. 14 - 14.71 Propose a structure consistent with each set...Ch. 14 - 14.72 Reaction of unknown A with forms...Ch. 14 - Prob. 14.75PCh. 14 - 14.74 -Annulene shows two signals in its ...Ch. 14 - 14.75 Explain why the spectrum of-methylbutan--ol...Ch. 14 - 14.76 Because has an odd mass number, nuclei...Ch. 14 - 14.77 Cyclohex--enone has two protons on its...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
The validity of a scientific law.
Physical Universe
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hand written equations pleasearrow_forwardHand written equations pleasearrow_forward> each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X Ś CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) © 2025 McGraw Hill LLC. All Rights Farrow_forward
- NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at 4.1 ppm? Select the single best answer. The H O HỌC—C—0—CH, CH, 2 A ethyl acetate H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm Check OA B OC ch B C Save For Later Submit Ass © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forwardHow many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red Note for advanced students: In this question, any multiplet is counted as one signal. 1 Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. O ✓ No additional Hs to color in top molecule ง No additional Hs to color in bottom…arrow_forwardin the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forward
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY