
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.18P
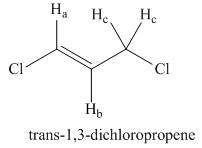
Draw a splitting diagram for
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.
The quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.
If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 14 - Prob. 14.2PCh. 14 - How many 1H NMR signals does each compound show?Ch. 14 - How many 1H NMR signals does each...Ch. 14 - Label the protons in each highlighted CH2 group as...Ch. 14 - How many 1H NMR signals would you expect for each...Ch. 14 - Prob. 14.7PCh. 14 - For each compound, first label each different type...Ch. 14 - Prob. 14.9PCh. 14 - Which compound give a 1H NMR spectrum with two...
Ch. 14 - Prob. 14.11PCh. 14 - Prob. 14.12PCh. 14 - Prob. 14.13PCh. 14 - For each compound give the number of 1H NMR...Ch. 14 - Prob. 14.15PCh. 14 - Prob. 14.16PCh. 14 - Describe the 1H NMR spectrum of each compound....Ch. 14 - Draw a splitting diagram for Hb in...Ch. 14 - Problem 14.20 Identify A and B, isomers of...Ch. 14 - Problem 14.21 How many signals are present in the ...Ch. 14 - What protons in alcohol A give rise to each signal...Ch. 14 - How many peaks are observed in the NMR signal for...Ch. 14 - Propose a structure for a compound of molecular...Ch. 14 - Problem 14.25 Propose a structure for a compound...Ch. 14 - Problem 14.26. Identify products A and B from the...Ch. 14 - How many lines are observed in the 13C NMR...Ch. 14 - Problem 14.28 Draw all constitutional isomers of...Ch. 14 - Esters of chrysanthemic acid are naturally...Ch. 14 - Prob. 14.29PCh. 14 - Problem 14.31 Identify the carbon atoms that give...Ch. 14 - Problem 14.32 A compound of molecular formula ...Ch. 14 - Problem 14.33 Draw the structure of a compound of...Ch. 14 - 14.34 (a) How many NMR signals does each of the...Ch. 14 - 14.35 (a) How many NMR signals does each compound...Ch. 14 - How many different types of protons are present in...Ch. 14 - How many 1H NMR signals does each compound give?...Ch. 14 - 14.37 How many NMR signals does each natural...Ch. 14 - Prob. 14.38PCh. 14 - Acetone exhibits a singlet in its 1H NMR spectrum...Ch. 14 - Prob. 14.40PCh. 14 - Prob. 14.41PCh. 14 - How could you use chemical shift and integration...Ch. 14 - Which compounds give one singlet in the 1H NMR...Ch. 14 - Prob. 14.44PCh. 14 - 14.43 How can you use NMR spectroscopy to...Ch. 14 - Prob. 14.46PCh. 14 - Prob. 14.47PCh. 14 - Which compounds in Problem 14.43 give one signal...Ch. 14 - Prob. 14.49PCh. 14 - How many 13C NMR signals does each compound...Ch. 14 - Rank the highlighted carbon atoms in each compound...Ch. 14 - 14.50 Identify the carbon atoms that give rise to...Ch. 14 - 14.51 a. How many signals does dimethyl...Ch. 14 - 14.53 Propose a structure consistent with each set...Ch. 14 - 14.54 Identify the structures of isomers A and B...Ch. 14 - 14.55 Reaction of with affords compound W,...Ch. 14 - 14.56 Treatment of with , followed by aqueous
...Ch. 14 - 14.57 Compound C has a molecular ion in its mass...Ch. 14 - 14.58 As we will learn in Chapter 20, reaction of ...Ch. 14 - 14.59 Identify the structures of isomers E and F...Ch. 14 - 14.59 Identify the structures of isomers H and I...Ch. 14 - 14.61 Propose a structure consistent with each set...Ch. 14 - 14.62 Reaction of with , followed by treatment...Ch. 14 - Reaction of aldehyde D with amino alcohol E in the...Ch. 14 - 14.64 Propose a structure consistent with each set...Ch. 14 - 14.65 In the presence of a small amount of acid, a...Ch. 14 - 14.66 Treatment of with affords two products (M...Ch. 14 - 14.67 Compound O has molecular formula and shows...Ch. 14 - 14.68 Compound P has molecular formula . Deduce...Ch. 14 - 14.69 Treatment of with strong base followed by ...Ch. 14 - Low molecular weight esters RCO2R often have...Ch. 14 - 14.70 When -bromo--dimethylbutane is treated with...Ch. 14 - 14.71 Propose a structure consistent with each set...Ch. 14 - 14.72 Reaction of unknown A with forms...Ch. 14 - Prob. 14.75PCh. 14 - 14.74 -Annulene shows two signals in its ...Ch. 14 - 14.75 Explain why the spectrum of-methylbutan--ol...Ch. 14 - 14.76 Because has an odd mass number, nuclei...Ch. 14 - 14.77 Cyclohex--enone has two protons on its...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forwardDoes Avogadro's number have units?arrow_forward
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY