
Each of the following alcohols is named incorrectly. However, the names give correct structural formulas. Draw structural formulas for the compounds, and then write the correct IUPAC name for each alcohol.
- a. 2-Ethyl-1-propanol
- b. 2,4-Butanediol
- c. 2-Methyl-3-butanol
- d. 1,4-Cyclopentanediol
(a)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
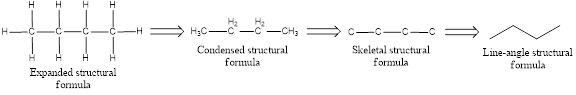
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
The structural formula is,
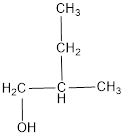
The correct IUPAC name of the given alcohol is 2-methyl-1-butanol.
Explanation of Solution
Given name of alcohol is 2-ethyl-1-propanol.
From the name it is identified that the parent alkane is propane with a hydroxyl group on first carbon atom and an ethyl group on second carbon atom.

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a four carbon chain and hence the parent is butane. As the structure has a hydroxyl group in it, the suffix “-ol” has to be added instead of “-e” in the parent alkane. The numbering has to be given in a way that the hydroxyl group gets the least numbering. Looking for the substituent, a methyl group is present on the second carbon atom. This gives the IUPAC name of the alcohol as 2-methyl-1-butanol as hydroxyl is in the first carbon atom.
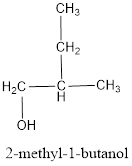
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
(b)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
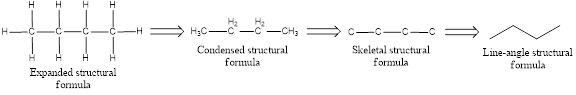
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
The structural formula is,
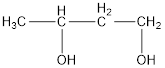
The correct IUPAC name of the given alcohol is 1,3-butanediol.
Explanation of Solution
Given name of alcohol is 2,4-butanediol.
From the name it is identified that there are two hydroxyl groups present each on second and fourth carbon atom of the parent alkane, butane. The structure can be drawn as,

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a four carbon chain and hence the parent is butane. As the structure has two hydroxyl groups in it, the suffix “-diol” has to be added to the name of parent alkane. The numbering has to be given in a way that the hydroxyl group gets the least numbering. This gives the IUPAC name of the alcohol as 1,3-butanediol.
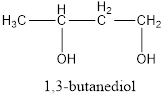
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
(c)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
The structural formula is,
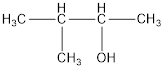
The correct IUPAC name of the given alcohol is 3-methyl-2-butanol.
Explanation of Solution
Given name of alcohol is 2-methyl-3-butanol.
From the name it is identified that the parent alkane is butane with a methyl group substituted in second carbon atom and a hydroxyl functional group on third carbon atom. The structure can be drawn as,

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a four carbon chain and hence the parent is butane. As the structure has a hydroxyl group in it, the suffix “-ol” has to be added instead of “-e” in the parent alkane. The numbering has to be given in a way that the hydroxyl group gets the least numbering. Looking for the substituents, a methyl group is present in the third carbon atom. This gives the IUPAC name of the alcohol as 3-methyl-2-butanol as hydroxyl is in the second carbon atom.
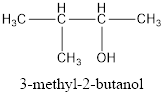
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
(d)
Interpretation:
Structural formula has to be written for the given alcohols and correct IUPAC name has to be assigned.
Concept Introduction:
The structural representation of organic compound can be done in 2D and 3D. In two-dimensional representation, there are four types of representation in which an organic compound can be drawn. They are,
- Expanded structural formula
- Condensed structural formula
- Skeletal structural formula
- Line-angle structural formula
Structural formula which shows all the atoms in a molecule along with all the bonds that is connecting the atoms present in the molecule is known as Expanded structural formula.
Structural formula in which grouping of atoms are done and in which the central atoms along with the other atoms are connected to them are treated as group is known as Condensed structural formula.
Structural formula that shows the bonding between carbon atoms alone in the molecule ignoring the hydrogen atoms being shown explicitly is known as Skeletal structural formula.
Structural formula where a line represent carbon‑carbon bond and the carbon atom is considered to be present in each point and the end of lines is known as Line-angle structural formula.
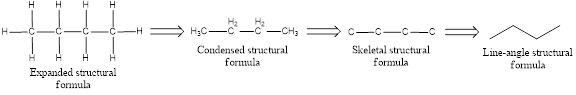
IUPAC rules for naming alcohols that contain single hydroxyl group:
- Longest carbon chain has to be identified that contains hydroxyl group also. The chain name is obtained by replacing the letter “-e” in alkane with “-ol”.
- The numbering has to be given so that the hydroxyl group gets the least numbering.
- Name and location of any other substituent present in the chain has to be identified.
- If in a ring the hydroxyl group is present, then that carbon is numbered 1 and the numbering then proceeds counterclockwise or clockwise in a way that substituents present if any gets the least numbering.
- Hydroxyl group as a substituent in a molecule is named as hydroxy group rather than hydroxyl group.
IUPAC rules for naming alcohols that contain more than one hydroxyl group:
- The same rules said above is followed but the prefix di-, tri-, tetra etc is added corresponding to the number of hydroxyl groups that is present.
Answer to Problem 14.19EP
The structural formula is,
The correct IUPAC name of the given alcohol is 1,3-cyclopentanediol.
Explanation of Solution
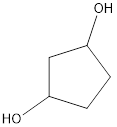
Given name of alcohol is 1,4-cyclopentanediol.
From the name it is identified that the parent cycloalkane is cyclopentane with two hydroxyl groups at first and fourth carbon atom. The structure can be drawn as,

The structural formula for the given alcohol is drawn as shown above.
IUPAC name can be identified by finding the longest continuous carbon chain with the hydroxyl group. In this case it is found to be a five carbon cyclic chain and hence the parent is cyclopentane. As the structure has two hydroxyl groups in it, the suffix “-diol” has to be added. The numbering has to be given in a way that the hydroxyl group gets the least numbering. This gives the IUPAC name of the alcohol as 1,3-cyclopentanediol as hydroxyl groups are in the first and third carbon atom.
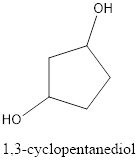
The structural formula for the given alcohol is drawn and correct IUPAC name is assigned.
Want to see more full solutions like this?
Chapter 14 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- A package contains 1.33lbs of ground round. If it contains 29% fat, how many grams of fat are in the ground? arrow_forwardHow is the resonance structure formed to make the following reaction product. Please hand draw the arrows showing how the electrons move to the correct position. Do not use an AI answer. Please draw it yourself or don't bother.arrow_forwardPart II Calculate λ max of the following compounds using wood ward- Fiecer rules a) b) c) d) e) OH OH dissolved in dioxane Br Br dissolved in methanol. NH₂ OCH 3 OHarrow_forward
- 6. Match each of the lettered items in the column on the left with the most appropriate numbered item(s) in the column on the right. Some of the numbered items may be used more than once and some not at all. a. Z = 37 1. b. Mn 2. C. Pr element in period 5 and group 14 element in period 5 and group 15 d. S e. [Rn] 7s¹ f. d block metal 3. highest metallic character of all the elements 4. paramagnetic with 5 unpaired electrons 5. 4f36s2 6. isoelectronic with Ca²+ cation 7. an alkaline metal 8. an f-block elementarrow_forwardDraw all formal charges on the structures below as is and draw 1 resonance structure that is more stable.arrow_forwardPart II. xiao isolated a compound TAD (Ca H 10 N₂) from tobacco and obtained its IR spectrum. Xiao proposed a chemical structure shown below: % Transmittance 4000 3500 3000 2500 2000 Wavenumber (cm-1) 1500 1000 (a) Explain why her proposed structure is inconsistent with the IR spectrum obtained (b) TAD exists as a tautomer of the structure xiao proposed. Draw the structure and explain why it is more compatible with the obtained spectrum. (C) what is the possible source for the fairly intense signal at 1621cm1arrow_forward
- AE>AE₁ (Y/N) AE=AE₁ (Y/N) AEarrow_forwardTreatment of 2-phenylpropan-2-amine with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. ? NH2 Br Br Propose a structural formula for compound A. You do not have to explicitly draw H atoms. You do not have to consider stereochemistry. In cases where there is more than one answer, just draw one. R3N C14H19NO2 + 2 R3NH*Br Aarrow_forwardCorrectly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardPart VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forwardDon't used Ai solutionarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





