
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13.3, Problem 13.8P
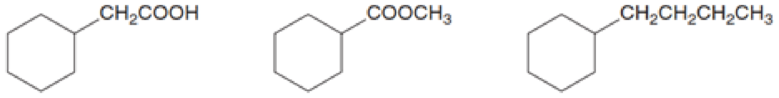
Rank the following compounds in order of increasing boiling point. Which compound is the most water soluble? Which compound is the least water soluble?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the two has a higher boiling point: Propanol or Pentanol?
Which of the following compounds has the highest water solubility?
ОН
which has the highest solubility between methanol and hexane?
Chapter 13 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 13.1 - Draw out each compound to clearly show what groups...Ch. 13.1 - Prob. 13.2PCh. 13.2 - Prob. 13.3PCh. 13.2 - Give the structure corresponding to each IUPAC...Ch. 13.2 - Prob. 13.5PCh. 13.2 - Give the structure corresponding to each name. a....Ch. 13.3 - Which compound in each pair has the higher boiling...Ch. 13.3 - Rank the following compounds in order of...Ch. 13.4 - Which compounds are -hydroxy acids? tartaric acid...Ch. 13.4 - Prob. 13.10P
Ch. 13.5 - Prob. 13.11PCh. 13.5 - Prob. 13.12PCh. 13.5 - Prob. 13.13PCh. 13.6 - Prob. 13.14PCh. 13.6 - Prob. 13.15PCh. 13.6 - Prob. 13.16PCh. 13.6 - Prob. 13.17PCh. 13.6 - Prob. 13.18PCh. 13.6 - Prob. 13.19PCh. 13.7 - Prob. 13.20PCh. 13.7 - Prob. 13.21PCh. 13.7 - Prob. 13.22PCh. 13.7 - Prob. 13.23PCh. 13.7 - Prob. 13.24PCh. 13.8 - Prob. 13.25PCh. 13.8 - Prob. 13.26PCh. 13.8 - Prob. 13.27PCh. 13.8 - Draw the product formed when each ammonium salt is...Ch. 13.8 - Prob. 13.29PCh. 13.9 - Prob. 13.30PCh. 13.9 - Prob. 13.31PCh. 13.9 - Prob. 13.32PCh. 13.9 - Why is the boiling point of CH3CONH2(221C) higher...Ch. 13.9 - Prob. 13.34PCh. 13.9 - Prob. 13.35PCh. 13.10 - Prob. 13.36PCh. 13 - Prob. 13.37UKCCh. 13 - Prob. 13.38UKCCh. 13 - Prob. 13.39UKCCh. 13 - Prob. 13.40UKCCh. 13 - Prob. 13.41UKCCh. 13 - Prob. 13.42UKCCh. 13 - Prob. 13.43UKCCh. 13 - Prob. 13.44UKCCh. 13 - Prob. 13.45UKCCh. 13 - Prob. 13.46UKCCh. 13 - Prob. 13.47UKCCh. 13 - Prob. 13.48UKCCh. 13 - Prob. 13.49UKCCh. 13 - Prob. 13.50UKCCh. 13 - Prob. 13.51APCh. 13 - Prob. 13.52APCh. 13 - Prob. 13.53APCh. 13 - Draw the structure of a compound of molecular...Ch. 13 - Prob. 13.55APCh. 13 - Prob. 13.56APCh. 13 - Give an acceptable name for each compound.Ch. 13 - Prob. 13.58APCh. 13 - Prob. 13.59APCh. 13 - Prob. 13.60APCh. 13 - Prob. 13.61APCh. 13 - Prob. 13.62APCh. 13 - Prob. 13.63APCh. 13 - Give an acceptable name for each amine or amide....Ch. 13 - Draw the structure corresponding to each name. a....Ch. 13 - Draw the structure corresponding to each name. a....Ch. 13 - Prob. 13.67APCh. 13 - Draw the structure of each amine or ammonium salt....Ch. 13 - Prob. 13.69APCh. 13 - Which compound in each pair is more water soluble?...Ch. 13 - Prob. 13.71APCh. 13 - Prob. 13.72APCh. 13 - Prob. 13.73APCh. 13 - Prob. 13.74APCh. 13 - Prob. 13.75APCh. 13 - Prob. 13.76APCh. 13 - Prob. 13.77APCh. 13 - Prob. 13.78APCh. 13 - Prob. 13.79APCh. 13 - Prob. 13.80APCh. 13 - Prob. 13.81APCh. 13 - Prob. 13.82APCh. 13 - Prob. 13.83APCh. 13 - Prob. 13.84APCh. 13 - Prob. 13.85APCh. 13 - Prob. 13.86APCh. 13 - Prob. 13.87APCh. 13 - Draw the products of each acid-base reaction.Ch. 13 - Prob. 13.89APCh. 13 - Prob. 13.90APCh. 13 - Prob. 13.91APCh. 13 - Prob. 13.92APCh. 13 - Ritalin is the trade name for methylphenidate, a...Ch. 13 - Prob. 13.94APCh. 13 - Prob. 13.95CPCh. 13 - Prob. 13.96CPCh. 13 - Prob. 13.97CPCh. 13 - Prob. 13.98BTCCh. 13 - Prob. 13.99BTCCh. 13 - Prob. 13.100BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Arrange the compounds in order of increasing boiling point. NH,arrow_forwardWrite the skeletal structures of propanal, acetone and cyclohexanone. What is the major intermolecular force (IMF) found in them? Based on their major intermolecular force and molecular weight, what can you predict on their solubility in water? Chemical Name Skeletal Structures Major IMF Solubility in water Propanal Acetone Cyclohexanonearrow_forwardRank the compounds in order of increasing melting point. OH Lowest Highestarrow_forward
- Which of these two would be more soluble in water? Why ?arrow_forwardDiethyl ether and butan-1-ol are isomers, and they have similar solubilities in water. Their boiling points are very different, however. Explain why these two compounds have similar solubility properties but dramatically different boiling points. CH3CH2¬O¬CH2CH3 CH3CH2CH2CH2¬OH diethyl ether, bp 35 °C butan-1-ol, bp 118 °C 8.4 mL dissolves in 100 mL H2O 9.1 mL dissolves in 100 mL H2Oarrow_forwardEach compound given in this problem is a common organic solvent. From each pair of compounds, select the solvent with the greater solubility in water.arrow_forward
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardMethanol is fairly volatile and evaporates quickly if spilled. Methanol is also absorbed quite readily through the skin. If, in the laboratory, methanol accidentally spilled on your clothing, why would it be a serious mistake to just let it evaporate?arrow_forwardArrange these compounds in order of increasing boiling point (values in C are 42, 24, 78, and 118). (a) CH3CH2OH (b) CH3OCH3 (c) CH3CH2CH3 (d) CH3COOHarrow_forward
- 14-67 Of the three compounds given in Problem 14-66, one is insoluble in water, another has a solubility of 2.3 g/100 g water, and one is infinitely soluble in water. Which compound has which solubility?arrow_forwardCompound A and compound B (see figure below) are used in different industries. They both have six carbon atoms. Which would be soluble in water and which would be soluble in hexane?arrow_forwardThe boiling points of the following compounds are as shown below although they have nearly equal molecular weights. Explain the difference between the three. Butan-1-ol (117°C), 1-chlorobutane (51°C), butanal (75°C)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY