
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 13.93AP
Ritalin is the trade name for methylphenidate, a drug used to treat attention deficit hyperactivity disorder (ADHD).
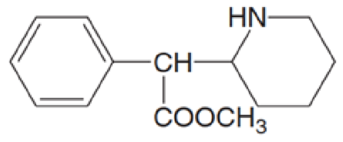
methylphenidate
(Trade name: Ritalin)
- a. Identify the
functional groups. - b. Label the
amine as 1°, 2°, or 3° - c. Draw the structure of methylphenidate hydrochloride.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
select two. Which statements correspond to:
ESCITALOPRAM
(antidepressant)
a. It induces tranquility and somnolence to the patient.
b. An agent that regulates re-uptake of serotonin in the brain.
c. Its structure has benzonitrile, flurobenzene and a tertiary amine.
d. An agent that is indicated to patients with hives and dermatitis.
What are the functional groups present in this antibacterial antibiotic?
A. Amide, thioether, aldehyde, phenol, carboxylic acid
B. Amide, thioether, ketone, amine, phenol, carboxylic acid
C. Amide, thioether, ketone, phenol, carboxylic acid
D. Thioether, ketone, amine, phenol, carboxylic acid
A brief explanation would be highly appreciated + upvote
The hydrolysis of an amide in acidic conditions forms
A. a carboxylate salt and an alcohol
B. a carboxylate salt and an amine
C. an alcohol and an amine salt (an ammonium ion)
D. a carboxylic acid and an amine salt (an ammonium ion)
Chapter 13 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 13.1 - Draw out each compound to clearly show what groups...Ch. 13.1 - Prob. 13.2PCh. 13.2 - Prob. 13.3PCh. 13.2 - Give the structure corresponding to each IUPAC...Ch. 13.2 - Prob. 13.5PCh. 13.2 - Give the structure corresponding to each name. a....Ch. 13.3 - Which compound in each pair has the higher boiling...Ch. 13.3 - Rank the following compounds in order of...Ch. 13.4 - Which compounds are -hydroxy acids? tartaric acid...Ch. 13.4 - Prob. 13.10P
Ch. 13.5 - Prob. 13.11PCh. 13.5 - Prob. 13.12PCh. 13.5 - Prob. 13.13PCh. 13.6 - Prob. 13.14PCh. 13.6 - Prob. 13.15PCh. 13.6 - Prob. 13.16PCh. 13.6 - Prob. 13.17PCh. 13.6 - Prob. 13.18PCh. 13.6 - Prob. 13.19PCh. 13.7 - Prob. 13.20PCh. 13.7 - Prob. 13.21PCh. 13.7 - Prob. 13.22PCh. 13.7 - Prob. 13.23PCh. 13.7 - Prob. 13.24PCh. 13.8 - Prob. 13.25PCh. 13.8 - Prob. 13.26PCh. 13.8 - Prob. 13.27PCh. 13.8 - Draw the product formed when each ammonium salt is...Ch. 13.8 - Prob. 13.29PCh. 13.9 - Prob. 13.30PCh. 13.9 - Prob. 13.31PCh. 13.9 - Prob. 13.32PCh. 13.9 - Why is the boiling point of CH3CONH2(221C) higher...Ch. 13.9 - Prob. 13.34PCh. 13.9 - Prob. 13.35PCh. 13.10 - Prob. 13.36PCh. 13 - Prob. 13.37UKCCh. 13 - Prob. 13.38UKCCh. 13 - Prob. 13.39UKCCh. 13 - Prob. 13.40UKCCh. 13 - Prob. 13.41UKCCh. 13 - Prob. 13.42UKCCh. 13 - Prob. 13.43UKCCh. 13 - Prob. 13.44UKCCh. 13 - Prob. 13.45UKCCh. 13 - Prob. 13.46UKCCh. 13 - Prob. 13.47UKCCh. 13 - Prob. 13.48UKCCh. 13 - Prob. 13.49UKCCh. 13 - Prob. 13.50UKCCh. 13 - Prob. 13.51APCh. 13 - Prob. 13.52APCh. 13 - Prob. 13.53APCh. 13 - Draw the structure of a compound of molecular...Ch. 13 - Prob. 13.55APCh. 13 - Prob. 13.56APCh. 13 - Give an acceptable name for each compound.Ch. 13 - Prob. 13.58APCh. 13 - Prob. 13.59APCh. 13 - Prob. 13.60APCh. 13 - Prob. 13.61APCh. 13 - Prob. 13.62APCh. 13 - Prob. 13.63APCh. 13 - Give an acceptable name for each amine or amide....Ch. 13 - Draw the structure corresponding to each name. a....Ch. 13 - Draw the structure corresponding to each name. a....Ch. 13 - Prob. 13.67APCh. 13 - Draw the structure of each amine or ammonium salt....Ch. 13 - Prob. 13.69APCh. 13 - Which compound in each pair is more water soluble?...Ch. 13 - Prob. 13.71APCh. 13 - Prob. 13.72APCh. 13 - Prob. 13.73APCh. 13 - Prob. 13.74APCh. 13 - Prob. 13.75APCh. 13 - Prob. 13.76APCh. 13 - Prob. 13.77APCh. 13 - Prob. 13.78APCh. 13 - Prob. 13.79APCh. 13 - Prob. 13.80APCh. 13 - Prob. 13.81APCh. 13 - Prob. 13.82APCh. 13 - Prob. 13.83APCh. 13 - Prob. 13.84APCh. 13 - Prob. 13.85APCh. 13 - Prob. 13.86APCh. 13 - Prob. 13.87APCh. 13 - Draw the products of each acid-base reaction.Ch. 13 - Prob. 13.89APCh. 13 - Prob. 13.90APCh. 13 - Prob. 13.91APCh. 13 - Prob. 13.92APCh. 13 - Ritalin is the trade name for methylphenidate, a...Ch. 13 - Prob. 13.94APCh. 13 - Prob. 13.95CPCh. 13 - Prob. 13.96CPCh. 13 - Prob. 13.97CPCh. 13 - Prob. 13.98BTCCh. 13 - Prob. 13.99BTCCh. 13 - Prob. 13.100BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- N-p-hydroxyphenylethanamide is commonly known as a. acetaminophen b. acetamide c. acetanilide d. formamide High molar mass amines have __________ odor. a.strong ammoniacal b.fruity c.fishy d.obnoxious Trimethyl amine has _________ odor. a.obnoxious b.fishy c. ammoniacal d. fruityarrow_forwardWhich of the following is an amine? Select one: a. HCONH2 b. CH3COCH3 c. CH3F d. CH3NH2arrow_forwardAminesarrow_forward
- 2. What is produced when an amine reacts with a strong acid such as HCl? A. An amine and the OH- ion B. An amide and the H+ ion C. An ammonium hydroxide D. An ammonium saltarrow_forwardJj.166. Draw each amide. N-butyl-N-methylbenzamidearrow_forwardMany drugs are sold as their hydrochloride salts (R2NH2+ Cl−), formed by reaction of an amine (R2NH) with HCl. a. Draw the product (a hydrochloride salt) formed by reaction of acebutolol with HCl. Acebutolol is a β blocker used to treat high blood pressure. b. Discuss the solubility of acebutolol and its hydrochloride salt in water. c. Offer a reason as to why the drug is marketed as a hydrochloride salt rather than a neutral amine.arrow_forward
- Many drugs are sold as their hydrochloride salts (R2NH2+ Cl−), formed by reaction of an amine (R2NH) with HCl. a.Draw the product (a hydrochloride salt) formed by reaction of acebutolol with HCl. Acebutolol is a β blocker used to treat high blood pressure. b. Discuss the solubility of acebutolol and its hydrochloride salt in water. c.Offer a reason as to why the drug is marketed as a hydrochloride salt rather than a neutral amine.arrow_forwardThis compound is mescaline, a hallucinogen. CHO CHO T Which of the following statements is true? Select one: O a. O b. O c. O d. Mescaline is an amine. Mescaline contains an alcohol group. Mescaline is a carboxylic acid. Mescaline is an amine and contains an alcohol group.arrow_forward1. Draw the structure for each compound and classify the amine as primary, secondary, or tertiary. a. dimethylamine b. diethylmethylamine c. 2-aminoethanolarrow_forward
- Why are tertiary amines not soluble in water? O a. Primary amine cannot hydrogen bond with water. O b. Amines are non polar compounds. O c. Tertiary amine cannot hydrogen bond with water O d. Secondary amine cannot hydrogen bond with water. Clear my choicearrow_forwardThe following are central stimulants: Select one or more: A. Barbiturates. B. Morphine. C. Benzodiazepines. D. Cocaine. E. Amphetamines.arrow_forwardQuinapril (trade name Accupril) is a drug used to treat hypertension and congestive heart failure.a.Identify the functional groups in quinapril. b.Classify any alcohol, amide, or amine as 1°, 2°, or 3°. c. At which sites can quinapril hydrogen bond to water? d.At which sites can quinapril hydrogen bond to acetone [(CH3)2CO]? e. Label the most acidic hydrogen atom. f.Which site is most basic?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY