
Concept explainers
Interpretation:
Whether Charles’s law can be used to determine absolute zero has to be explained.
Concept Introduction:
Charles’s law: The volume of a given amount of gas is proportional to its temperature (in K) of the gas at constant pressure.
The relationship between volume of a gas and temperature is expressed as-
Answer to Problem 13A
If the lines in volume vs. temperature in Kelvin graph extrapolate to zero volume at the same temperature which is -273 °C. This temperature -273 °C is the lowest possible
Explanation of Solution
Charles’s law: The volume of a given amount of gas is proportional to its temperature (in K) of the gas at constant pressure.
The relationship between volume of a gas and temperature is expressed as-
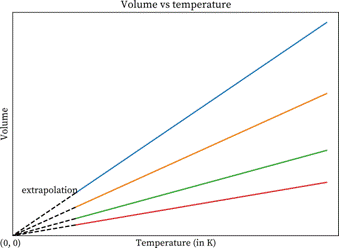
If the lines in volume vs. temperature in Kelvin graph extrapolate to zero volume at the same temperature which is -273 °C. This temperature -273 °C is the lowest possible thermodynamic temperature when when the volume of a gas is zero. Therefore, this temperature is defined as absolute zero on the Kelvin scale which is derived from the Charles’s law.
Chapter 13 Solutions
World of Chemistry, 3rd edition
- Part C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forwardPart D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forwardPart B: The line formula for a branched alkane is shown below. a. What is the molecular formula of this compound? Number of C. Number of H b. How many carbon atoms are in the longest chain? c. How many alkyl substituents are attached to this chain?arrow_forward
- 24. What is the major product for the following reaction? Mg J. H.C CH H,C- Then H₂O OH Br C HO E HO H.C CH H.C- CH₂ CH₂ All of these are possiblearrow_forwardstructures. Explain why the major product(s) are formed over the minor product(s) using the Draw the major and product and the complete mechanism for all products with all resonance mechanism/resonance structures of the major and minor products in your explanation. HONO2 H2SO4arrow_forward#1 (a). Provide the expected product for the following reaction of A to B by indicating what the product is after step 1 (call this "81") and after step 2 (call this product "B2"). Give a complete mechanism for the transformation of compound A into compound B showing all intermediates, resonance structures, stereochemistry and electron movements 1. Et-MgBr 2. Me-Br B #1 (b). Compound A can be prepared in one step from an alkene starting material. Provide the structure a and the reaction conditions required to convert it to compound A The starting alkenearrow_forward
- The line formula for a branched alkene is shown below. 2 i. What is the molecular formula of this compound? Count number of C and H ii. How many carbon atoms are in the longest chain, ignoring the double bond? iii. What is the longest chain incorporating both carbons of the double bond? iv. How many substituents are on this chain? v. Give the IUPAC name for this compoundarrow_forwardgive the products for each of the followingarrow_forwardProvide the products and/or reagents for the following transformations. NaOMe HCl/EtOH OH NaOMe CI Show the product for the formation of the ketal given below for the transformation, showing all intermediates and resonance structures would be required to transform the ketal back to the starting ketone and then the mechanism What reagents/conditions HCI EtOH (excess)arrow_forward
- Make meta-dibromobenze from nitrobenzene using amine reactions. *see imagearrow_forwardProvide the structure of the expected major and minor (if any) products for each reaction. Clearly indicate stereochemistry where warranted. + + heat heat 이요 HNO3 1. AlCl3 2. H₂O H2SO4 1. AlCl3arrow_forward) Give the mechanism for the acid catalyzed hydrolysis of the following to the corresponding carboxylic acid. Show all intermediates and resonance structures N H+, H2O (excess)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





