
Concept explainers
(a)
Interpretation:
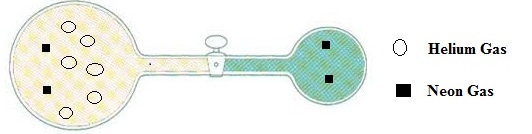
For the given diagram of helium and neon gases, which gas has greater initial pressure and how much pressure it has, is to be identified.
Concept Introduction :
- The ideal gases are those gases which obey the ideal gas equation and the ideal gas equation is written as
Where, P = pressure of the gas
V = Volume of the gas
R = Gas constant
T = temperature of the
- Boyle’s Law gives the inverse relation of volume with pressure at constant temperature and number of moles, such that
(a)
Answer to Problem 65A
The initial pressure of helium gas is 1.5 times greater than the initial pressure of neon gas.
Explanation of Solution
Given information:
The number of molecules of helium gas =
The number of molecules of neon gas = 4
Calculation:
The relation between pressure and number of moles of gases at the same temperature and volume is
So, the initial pressure of helium gas is greater than the initial pressure of neon gas.
(b)
For the diagram of helium and neon gases, the diagram will looks after opened the stopcock
By neglecting the volume of the connecting tube is to be drawn.
Interpretation:
Concept Introduction :
The ideal gases are those gases which obey the ideal gas equation and the ideal gas equation is written as
Where, P = pressure of the gas
V = Volume of the gas
R = Gas constant
T = temperature of the gas
- The inverse relation of volume with pressure at constant temperature and number of moles, such that
(b)
Answer to Problem 65A
The diagram after opened the stopcock is
Explanation of Solution
When the valve is opened, due to the pressure differences some moles of neon gas are moving from the right side bulb to the left side bulb.
(c)
Interpretation:
For the given diagram of helium and neon gases, the final pressure in terms of the original pressure of helium and neon gases is to be determined.
Concept Introduction :
- The ideal gases are those gases which obey the ideal gas equation and the ideal gas equation is written as
Where, P = pressure of the gas
V = Volume of the gas
R = Gas constant
T = temperature of the gas
- Boyle’s Law gives the inverse relation of volume with pressure at constant temperature and number of moles, such that
(c)
Answer to Problem 65A
The final pressure in terms of the original pressures of helium and neon at constant temperature is
Explanation of Solution
Given information:
The number of molecules of helium gas =
The number of molecules of neon gas = 4
The volume of each bulb =
Calculation:
Total volume =
The final pressure in terms of original pressure of neon gas
The final pressure in terms of original pressure of helium gas is
(d)
Interpretation:
For the given diagram of helium and neon gases, the final partial pressure of each gas in terms of the final pressure of the system is to be determined.
Concept Introduction :
- The ideal gases are those gases which obey the ideal gas equation and the ideal gas equation is written as
Where, P = pressure of the gas
V = Volume of the gas
R = Gas constant
T = temperature of the gas
- Boyle’s Law gives the inverse relation of volume with pressure at constant temperature and number of moles, such that
(d)
Answer to Problem 65A
The initial pressure of helium gas is 1.5 times greater than the initial pressure of neon gas.
Explanation of Solution
Given information:
The Initial number of molecules of helium gas =
The Initial number of molecules of neon gas = 4
Calculation:
The final partial pressure of helium gas in terms of final pressure
The final partial pressure of neon gas in terms of final pressure of the system
Chapter 13 Solutions
World of Chemistry, 3rd edition
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





