
Concept explainers
(a)
Interpretation:
The spatial arrangement for the
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Saturated hydrocarbons are
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(a)
Answer to Problem 13.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(b)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(b)
Answer to Problem 13.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(c)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(c)
Answer to Problem 13.37EP
The spatial arrangement is identified as trigonal planar.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is bonded to one double bond. This carbon atom has only two single bonds with hydrogen and a double bond with carbon atom. Therefore, the spatial arrangement of the left-most carbon atom is trigonal planar.
The spatial arrangement of the left-most carbon atom is identified.
(d)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(d)
Answer to Problem 13.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,
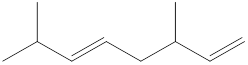
Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
Want to see more full solutions like this?
Chapter 13 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry A. CH₂OH PCC CH2Cl2 HOO B. H KCN HCN of b C. 1. CH,MgBr, ether 2 HO* D. Choose the BEST reagent for carrying out each of the following conversions. CO₂CH3 CO₂CH3 OH CO₂H сон ن نے a. LiAlH4, ether at abinayo iss c b. NaBH4, ethanol C. CrO3, pyridine d. H₂/Pd d notsiolarrow_forwardChoose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Use only one letter per box. OH OH CH CH CH3 CHS CH3 f OH OCH 3 H A. NaH, then CHI B. C. m-ClC6H4COзH D. E. warm H2SO4/H₂O F. G. H₂/Pd H. I. Cl₂, H₂O J. NaOCH3, CH3OH CH3MgBr in ether, then H3O+ Hg(O2CCF3)2, CH3OH PCC, CH2Cl2 LiAlH4 in ether, then H3O+arrow_forwardWhat is the product of the reaction of 2,4-pentanedione with phenylhydrazine?arrow_forward
- In the reaction of naphthalene with CrO3 in acetic acid. Indicate whether a different product is obtained if carried out at 25°C or with heating (A).arrow_forwardQUESTION: Fill in the answers in the empty green boxes 1. Step 2 2. Step 3 3. Step 4 (SUM) 4. Step 5 (df) (GIVEN) 5. Determine S y/x value *The data values have been provided in the worksheet attached in the first image*arrow_forwardIf the symbol A is placed in a reaction, at what temperature does it take place?arrow_forward
- By malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forwardoalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forward
- What type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





