
Anethole, the major constituent of anise oil, is used in licorice-flavored sweets and flavored brandy. Answer the following questions using the ball-and-stick model of anethole.
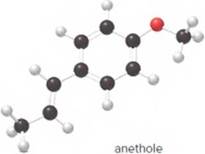
- What is the molecular formula of anethole?
(a)
Interpretation:
The molecular formula of anethole should be determined.
Concept Introduction:
The numbers and kinds of atoms present in a molecule of a compound are given by a formula said to be a molecular formula. This formula of a molecule gives the definite number of atoms of each element present in the compound.
Answer to Problem 13.29P
The molecular formula of anethole is C10H12O.
Explanation of Solution
The given ball-and-stick model of anethole is:
In ball-and-stick model, the black ball represents carbon, C atom, white ball represents hydrogen, H atom, and red ball represents oxygen, O atom.
The number of each atom in the ball-and-stick model is:
Carbon atoms = 10
Hydrogen atoms = 12
Oxygen atom = 1
Thus, the molecular of anethole is C10H12O.
(b)
Interpretation:
The functional group(s) in anethole should be determined.
Concept Introduction:
A group of atoms or an atom which is responsible for the characteristic reactions of a particular compound is said to be the functional group. Every functional group shows distinctive chemical properties irrespective to the moiety to which it is attached.
Answer to Problem 13.29P
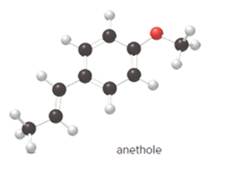
The functional group(s) in anethole is ether.
Explanation of Solution
The given ball-and-stick model of anethole is:
When the oxygen atom is connected to two alkyl or aryl groups with general formula R-O-R' such class of compounds is known as ether.
Since, in anethole compound the oxygen atom is bonded to an aryl and an alkyl group so, the functional group(s) in anethole is ether.
(c)
Interpretation:
The carbon-carbon double bond in anethole should be labeled as cis or trans.
Concept Introduction:
The molecule with same molecular formula and connectivity but differ in spatial arrangements of atoms in the molecule are said to be geometric isomers of each other.
Answer to Problem 13.29P
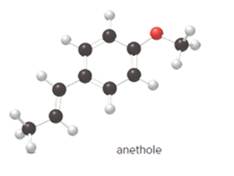
The carbon-carbon double bond in anethole is labeled as trans.
Explanation of Solution
The given ball-and-stick model of anethole is:
Geometric isomerism, or cis-trans, isomerism is most common in alkenes. The two forms exist due to no free rotation about carbon-carbon double bond. It exists only when the double bonded carbon atoms are joined to two different groups or atoms.
In cis- isomer, the two identical groups or atoms are close to each other whereas in trans- isomer two identical groups or atoms are farther apart.
Since, in the given molecule the groups attached to carbon-carbon double bonded are farther apart from each other so, the carbon-carbon double bond in anethole is trans.
(d)
Interpretation:
The structure of anethole should be drawn and each carbon should be labeled as trigonal planar or tetrahedral.
Concept Introduction:
In structural formula, the bonding and type of bonds which holds the atoms in molecule together are shown.
Answer to Problem 13.29P
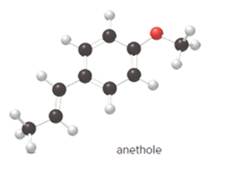
The structure of anethole is:
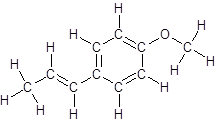
Labelling of each carbon is:
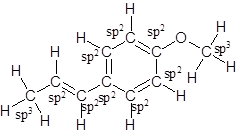
Explanation of Solution
The given ball-and-stick model of anethole is:
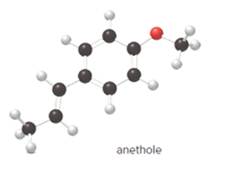
In ball-and-stick model, the black ball represents carbon, C atom, white ball represents hydrogen, H atom, and red ball represents oxygen, O atom.
So, the structure of anethole is:
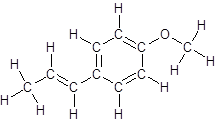
In the structure of anethole, there are 10 C atoms, 2 of which them are in CH3- groups which has four electron groups around the central carbon atom, so the electron group arrangement is tetrahedral, sp3hybridized. The 8 carbon atoms have three electron groups around the central carbon atom, so the electron group arrangement is trigonal planar, sp2hybridized.
Thus, the electron group arrangement of all the 10 carbon atoms in anethole is:
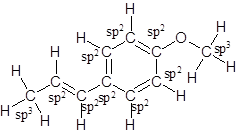
Want to see more full solutions like this?
Chapter 13 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





