
Concept explainers
Assign each of the compounds in Problem 13-108 an IUPAC name in which the substituents on the benzene ring are located using the ortho-, meta-, para-prefix system.
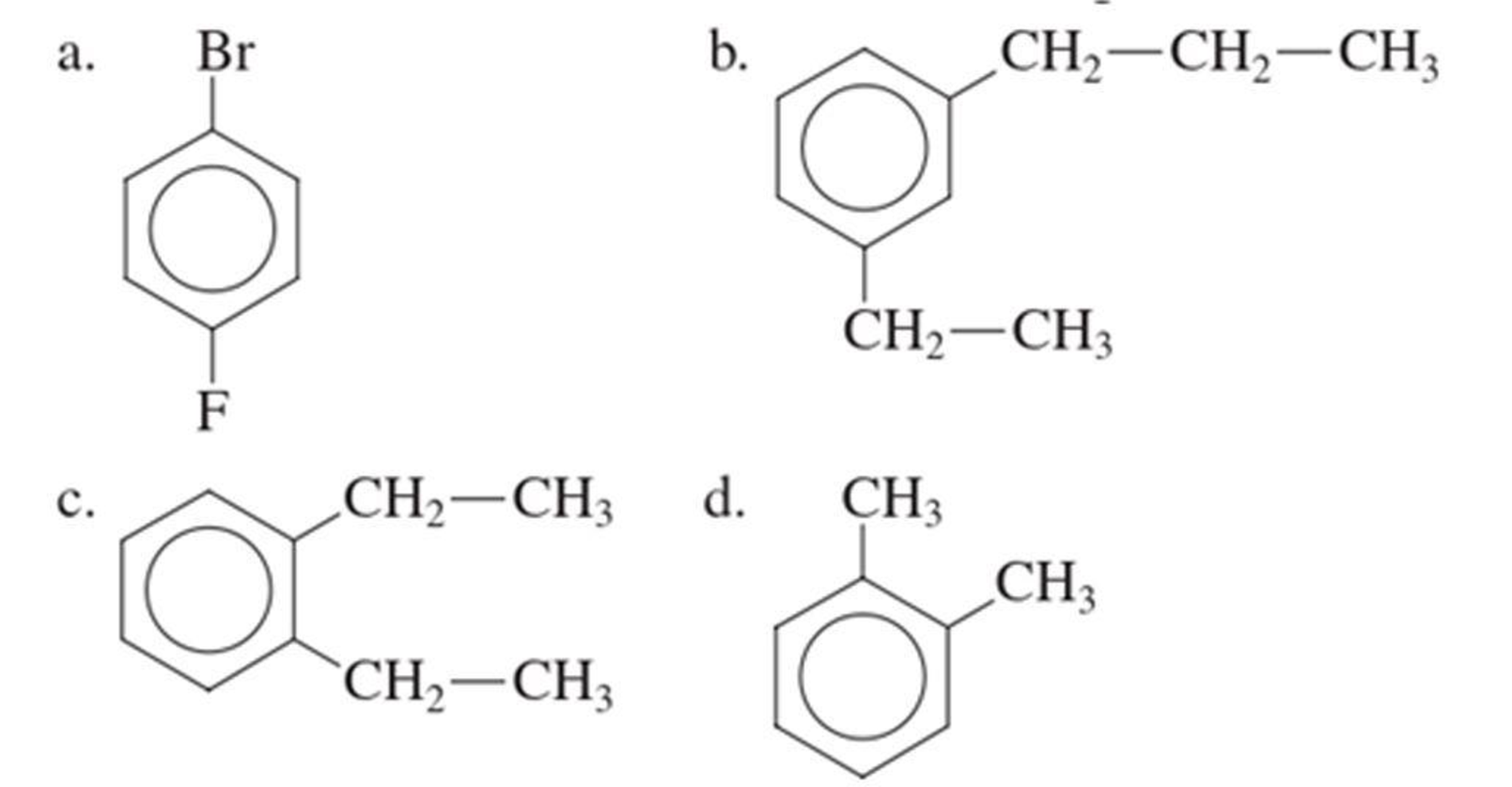
(a)
Interpretation:
The IUPAC name has to be given for the disubstituted benzene derivative by using ortho-, meta-, para- prefix system.
Concept Introduction:
When hydrogen atoms are replaced by one or more groups in benzene is known as substitution reaction and the compounds produced is benzene derivatives.
Benzene derivative with one substituent:
IUPAC system of naming monosubstituted benzene derivatives uses the name of substituent as prefix to the name benzene. If the group that is present in benzene cannot be named easily means, then the benzene ring is often treated as group attached to this substituent. The benzene ring is known as phenyl in this approach.
Benzene derivative with two substituents:
When benzene ring contains two substituents it is known as disubstituted benzene derivative. Three isomers are possible for the disubstituted benzene derivative. The prefix used in IUPAC name are,
Ortho- means disubstitution in 1,2
Meta- means disubstitution in 1,3
Para- means disubstitution in 1,4
When both the substituents present on the benzene ring imparts a special name, where all the substituents are cited in alphabetical order before the ending –benzene. The carbon that bears the group with alphabetical priority is given number 1.
Benzene derivatives with three or more substituents:
More than two groups are present in the benzene ring means, their positions are numbered. The numbering is always done in a way that the carbon atom bearing substituent gets the lowest numbering possible. If there is a choice of numbering system, then the group that comes alphabetically first is given the lowest number.
Answer to Problem 13.110EP
The IUPAC name of the given compound is p-bromofluorobenzene.
Explanation of Solution
Given structure is,

From the structure given above, it is found a fluorine atom and a bromine atom are present as substituent. The numbering has to be given so that the substituents get the least numbering. The numbering has to be given considering the alphabetical order of the substituents. This puts the bromine in number 1 and fluorine in number 4. As the substituents are present in first and fourth position, prefix para- can be used to name the compound. Therefore, the IUPAC name can be given as p-bromofluorobenzene.
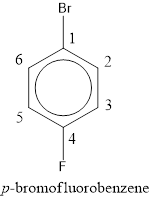
The IUPAC name can be given as p-bromofluorobenzene.
The correct IUPAC name for the given compound is given.
(b)
Interpretation:
The IUPAC name has to be given for the disubstituted benzene derivative by using ortho-, meta-, para- prefix system.
Concept Introduction:
When hydrogen atoms are replaced by one or more groups in benzene is known as substitution reaction and the compounds produced is benzene derivatives.
Benzene derivative with one substituent:
IUPAC system of naming monosubstituted benzene derivatives uses the name of substituent as prefix to the name benzene. If the group that is present in benzene cannot be named easily means, then the benzene ring is often treated as group attached to this substituent. The benzene ring is known as phenyl in this approach.
Benzene derivative with two substituents:
When benzene ring contains two substituents it is known as disubstituted benzene derivative. Three isomers are possible for the disubstituted benzene derivative. The prefix used in IUPAC name are,
Ortho- means disubstitution in 1,2
Meta- means disubstitution in 1,3
Para- means disubstitution in 1,4
When both the substituents present on the benzene ring imparts a special name, where all the substituents are cited in alphabetical order before the ending –benzene. The carbon that bears the group with alphabetical priority is given number 1.
Benzene derivatives with three or more substituents:
More than two groups are present in the benzene ring means, their positions are numbered. The numbering is always done in a way that the carbon atom bearing substituent gets the lowest numbering possible. If there is a choice of numbering system, then the group that comes alphabetically first is given the lowest number.
Answer to Problem 13.110EP
The IUPAC name of the given compound is m-ethylpropylbenzene.
Explanation of Solution
Given structure is,
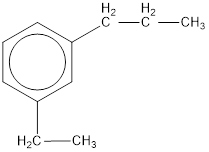
From the structure given above, it is found an ethyl group and a propyl group is present as substituents. The numbering has to be given so that the substituents get the least numbering. The numbering has to be given considering the alphabetical order of the substituents. This puts the ethyl in number 1 and propyl in number 3. As the susbtituents are present in first and third carbon atom, meta- can be used in the name as prefix. Therefore, the IUPAC name can be given as m-ethylpropylbenzene.
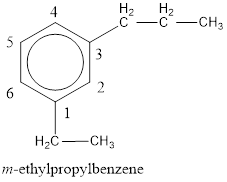
The IUPAC name can be given as m-ethylpropylbenzene.
The correct IUPAC name for the given compound is given.
(c)
Interpretation:
The IUPAC name has to be given for the disubstituted benzene derivative by using ortho-, meta-, para- prefix system.
Concept Introduction:
When hydrogen atoms are replaced by one or more groups in benzene is known as substitution reaction and the compounds produced is benzene derivatives.
Benzene derivative with one substituent:
IUPAC system of naming monosubstituted benzene derivatives uses the name of substituent as prefix to the name benzene. If the group that is present in benzene cannot be named easily means, then the benzene ring is often treated as group attached to this substituent. The benzene ring is known as phenyl in this approach.
Benzene derivative with two substituents:
When benzene ring contains two substituents it is known as disubstituted benzene derivative. Three isomers are possible for the disubstituted benzene derivative. The prefix used in IUPAC name are,
Ortho- means disubstitution in 1,2
Meta- means disubstitution in 1,3
Para- means disubstitution in 1,4
When both the substituents present on the benzene ring imparts a special name, where all the substituents are cited in alphabetical order before the ending –benzene. The carbon that bears the group with alphabetical priority is given number 1.
Benzene derivatives with three or more substituents:
More than two groups are present in the benzene ring means, their positions are numbered. The numbering is always done in a way that the carbon atom bearing substituent gets the lowest numbering possible. If there is a choice of numbering system, then the group that comes alphabetically first is given the lowest number.
Answer to Problem 13.110EP
The IUPAC name of the given compound is o-diethylbenzene.
Explanation of Solution
Given structure is,
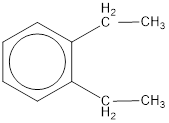
From the structure given above, it is found two ethyl groups are present as substituent. The numbering has to be given so that the substituents get the least numbering. This puts one ethyl group in position 1 and another ethyl group in position 2. As the groups substituted are identical, alphabetical order does not play a part but prefix di- has to be added. The groups that are substituted is present in first and second carbon atom. Hence, prefix ortho- can be used to name the compound. Therefore, the IUPAC name can be given as o-diethylbenzene.
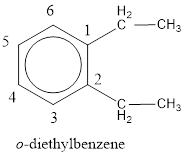
The IUPAC name can be given as o-diethylbenzene.
The correct IUPAC name for the given compound is given.
(d)
Interpretation:
The IUPAC name has to be given for the disubstituted benzene derivative by using ortho-, meta-, para- prefix system.
Concept Introduction:
When hydrogen atoms are replaced by one or more groups in benzene is known as substitution reaction and the compounds produced is benzene derivatives.
Benzene derivative with one substituent:
IUPAC system of naming monosubstituted benzene derivatives uses the name of substituent as prefix to the name benzene. If the group that is present in benzene cannot be named easily means, then the benzene ring is often treated as group attached to this substituent. The benzene ring is known as phenyl in this approach.
Benzene derivative with two substituents:
When benzene ring contains two substituents it is known as disubstituted benzene derivative. Three isomers are possible for the disubstituted benzene derivative. The prefix used in IUPAC name are,
Ortho- means disubstitution in 1,2
Meta- means disubstitution in 1,3
Para- means disubstitution in 1,4
When both the substituents present on the benzene ring imparts a special name, where all the substituents are cited in alphabetical order before the ending –benzene. The carbon that bears the group with alphabetical priority is given number 1.
Benzene derivatives with three or more substituents:
More than two groups are present in the benzene ring means, their positions are numbered. The numbering is always done in a way that the carbon atom bearing substituent gets the lowest numbering possible. If there is a choice of numbering system, then the group that comes alphabetically first is given the lowest number.
Answer to Problem 13.110EP
The IUPAC name of the given compound is o-dimethylbenzene.
Explanation of Solution
Given structure is,
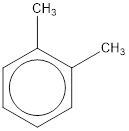
From the structure given above, it is found two methyl groups are present as substituent. The numbering has to be given so that the substituents get the least numbering. This puts one methyl group in position 1 and another methyl group in position 2. As the groups substituted are identical, alphabetical order does not play a part but prefix di- has to be added. The groups that are substituted is present in first and second carbon atom. Hence, prefix ortho- can be used to name the compound. Therefore, the IUPAC name can be given as o-dimethylbenzene.
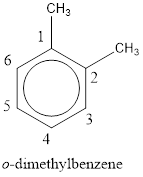
The IUPAC name can be given as o-dimethylbenzene.
The correct IUPAC name for the given compound is given.
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, and Biological Chemistry
- Don't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forward
- Show work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forwardBr. COOH Br, FCH COOH E FeBr ASOCI B NH (CH,CO),OD Br₂ 2 C alcKOHarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





