
Concept explainers
(a)
Interpretation:
The spatial arrangement for the
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Saturated hydrocarbons are
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(a)
Answer to Problem 13.38EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(b)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(b)
Answer to Problem 13.38EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(c)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(c)
Answer to Problem 13.38EP
The spatial arrangement is identified as trigonal planar.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is bonded to one double bond. This carbon atom has only two single bonds with hydrogen and a double bond with carbon atom. Therefore, the spatial arrangement of the left-most carbon atom is trigonal planar.
The spatial arrangement of the left-most carbon atom is identified.
(d)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(d)
Answer to Problem 13.38EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,
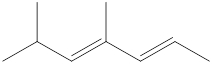
Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, and Biological Chemistry
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





