
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
12th Edition
ISBN: 9781337915977
Author: Bettelheim
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 24P
If you examine the structural formulas for the following cycloalkenes, you will see that the configuration of the double bond is cis in each.
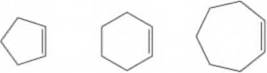
Cyclopentene Cyclohexene Cycloheptene
All attempts to synthesize these cycloalkenes in which the double bond has a trans configuration have failed. Apparently, it is impossible to have a
trans configuration in these cycloalkenes. Offer an explanation for why this is so.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part II. count the expected number of signals in the 1H-NMR spectrum of these compounds
HO
0
одев
*
Cl
-cl
"D"
Part I. Create a splitting tree diagram to predict the multiplet pattern of proton Hb in the compound below:
3
(Assume that "Jab >>> ³JbC)
Ha Hb He
он
Ha
NH2
Ha
HC
None
Chapter 12 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
Ch. 12.3 - Prob. 12.1QCCh. 12.3 - Prob. 12.2QCCh. 12.3 - Problem 12-3 Write the IUPAC name for each...Ch. 12.3 - Problem 12-4 Draw structural formulas for the...Ch. 12.3 - Problem 12-5 How many stereoisomers are possible...Ch. 12.5 - Prob. 12.6QCCh. 12.5 - Problem 12-7 Propose a two-step mechanism for the...Ch. 12.5 - Prob. 12.8QCCh. 12.5 - Problem 12-9 Propose a three-step reaction...Ch. 12.5 - Prob. 12.10QC
Ch. 12.8 - Prob. 12.11QCCh. 12 - Prob. 1PCh. 12 - Answer true or false. Both ethylene and acetylene...Ch. 12 - 12-13 What is the difference in structure between...Ch. 12 - There are three compounds with the molecular...Ch. 12 - 12-15 Name and draw structural formulas for all...Ch. 12 - Prob. 6PCh. 12 - Draw a structural formula for at least one...Ch. 12 - Each carbon atom in ethane and in ethylene is...Ch. 12 - Prob. 9PCh. 12 - Prob. 10PCh. 12 - Prob. 11PCh. 12 - 12*22 Draw a structural formula for each compound....Ch. 12 - 12-23 Draw a structural formula for each compound....Ch. 12 - Prob. 14PCh. 12 - 12-25 Write the IUPAC name for each unsaturated...Ch. 12 - Explain why each name is incorrect and then write...Ch. 12 - 12-27 Explain why each name is incorrect and then...Ch. 12 - Prob. 18PCh. 12 - 12-29 Which of these alkenes show cis-trans...Ch. 12 - 12-30 Which of these alkenes shows cis-trans...Ch. 12 - 12-31 Cyclodecene exists as both cis and trans...Ch. 12 - Arachidonic acid is a naturally occurring C„o...Ch. 12 - Prob. 23PCh. 12 - If you examine the structural formulas for the...Ch. 12 - 12*35 For each molecule that shows eis-trans...Ch. 12 - Name and draw structural formulas for all...Ch. 12 - /3-Ocimene, a triene found in the fragrance of...Ch. 12 - Answer true or false. Alkenes and alkynes are...Ch. 12 - Prob. 29PCh. 12 - 12-40 Define alkene addition reaction. Write an...Ch. 12 - Prob. 31PCh. 12 - 12-42 Complete these equations.Ch. 12 - Draw structural formulas for all possible...Ch. 12 - Prob. 34PCh. 12 - 12-45 Draw a structural formula for the product of...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - 12-47 Draw a structural formula for an alkene with...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - Prob. 39PCh. 12 - 12-50 Draw the structural formula of an alkene...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Following is the structural formula of...Ch. 12 - Propose an explanation for the following...Ch. 12 - There are nine alkenes with the molecular formula...Ch. 12 - Prob. 46PCh. 12 - 12-57 Hydrocarbon A, Cf,Hs, reacts with 2 moles of...Ch. 12 - 12-58 Show how to convert ethylene to these...Ch. 12 - 12-59 Show how to convert 1-butene to these...Ch. 12 - Prob. 50PCh. 12 - 12-61 (Chemical Connections 12A) What is one...Ch. 12 - Prob. 52PCh. 12 - Prob. 53PCh. 12 - 12-64 (Chemical Connections 120 What is the...Ch. 12 - (Chemical Connections 120 Assume that 1 X IO-12 g...Ch. 12 - Prob. 56PCh. 12 - Prob. 57PCh. 12 - Prob. 58PCh. 12 - Prob. 59PCh. 12 - Prob. 60PCh. 12 - Prob. 61PCh. 12 - Suppose you have unlabeled bottles of benzene and...Ch. 12 - Prob. 63PCh. 12 - Prob. 64PCh. 12 - Answer true or false. (a) Phenols and alcohols...Ch. 12 - Prob. 66PCh. 12 - Prob. 67PCh. 12 - Prob. 68PCh. 12 - Prob. 69PCh. 12 - 12-67 (Chemical Connections 12D ) In which isomer...Ch. 12 - Prob. 71PCh. 12 - Prob. 72PCh. 12 - Prob. 73PCh. 12 - Prob. 74PCh. 12 - Prob. 75PCh. 12 - Prob. 76PCh. 12 - Prob. 77PCh. 12 - Prob. 78PCh. 12 - Prob. 79PCh. 12 - Prob. 80PCh. 12 - Prob. 81PCh. 12 - Prob. 82PCh. 12 - Prob. 83PCh. 12 - Prob. 84PCh. 12 - Prob. 85PCh. 12 - Prob. 86PCh. 12 - Propose a structural formula for the product!s)...Ch. 12 - Prob. 88PCh. 12 - Draw the structural formula of an alkene that...Ch. 12 - 12-77 Show how to convert cyclopentene into these...Ch. 12 - Prob. 91PCh. 12 - Prob. 92PCh. 12 - Prob. 93PCh. 12 - Prob. 94PCh. 12 - Prob. 95PCh. 12 - In omega-3 fatty adds, the last carbon of the last...Ch. 12 - Prob. 97PCh. 12 - Prob. 98P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- SH 0arrow_forward2. Please consider the two all 'cis' isomers of trimethylcyclohexane drawn below. Draw the two chair conformers of each stereoisomer below (1 and 2) and calculate their torsional interaction energies in order to identify the lower energy conformer for each stereoisomer. Based on your calculations, state which of the two stereoisomers 1 and 2 is less stable and which is more stable. [1,3-diaxial CH3 CH3 = 3.7kcal/mol; 1,3-diaxial CH3 H = 0.88kcal/mol; cis-1,2 (axial:equatorial) CH3 CH3 = 0.88kcal/mol; trans-1,2-diequatorial CH3 CH3 = 0.88kcal/mol) all-cis-1,2,3- 1 all-cis-1,2,4- 2arrow_forwardNonearrow_forward
- What is the mechanism by which the 1,4 product is created? Please draw it by hand with arrows and stuff.arrow_forwardWhat is the relationship between A and B? H3C A Br Cl H3C B Br relationship (check all that apply) O same molecule O enantiomer O diastereomer structural isomer O stereoisomer isomer O need more information to decide O same molecule ☐ enantiomer Br Br Br CH3 Br CI CH3 O diastereomer ☐ structural isomer ☐ stereoisomer isomer O need more information to decide O same molecule O enantiomer Odiastereomer structural isomer O stereoisomer ☐ isomer O need more information to decidearrow_forwardb. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License