
Concept explainers
(a)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Answer to Problem 29P
The statement istrue.
Explanation of Solution
Alkenes are unsaturated hydrocarbons with carbon-carbon double bonds. The combustion reaction takes place in the presence of air. The combustion of alkenes produces carbon dioxide and water.
Hence, the given statement is true.
(b)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
The addition reaction of alkenes is one double bond of alkene and one single bond of a second reactant break, and three new single bonds are formed such that the addition of the second reactant occurs at the carbon atoms of the double bond. Hence, the two new single bonds are formed at the doubly bonded carbon atoms. Therefore, the given statement is true.
(c)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
Regioselectivity means the preference of making or breaking of bonds over all other possibilities. Markovnikov's rule states that in the addition of a halogen acid to an alkene, hydrogen goes to the carbon atom in a double bond that bears a higher number of hydrogen atoms. Thus, it indicates the position of the attack. Hence, Markovnikov's rule refers to the regioselectivity of the carbon-carbon double bond. Therefore, the given statement is true.
(d)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
Markovnikov's rule states that addition of a halogen acid (HX, x = Cl, Br or I) to an alkene, hydrogen goes to the carbon atom in the double bond that bears a higher number of hydrogen atoms. In additionof HCI, HBr, and HI to an alkene, hydrogen attaches to the carbon of the double bond that already has a greater number of hydrogen atoms bonded to it. Therefore, the given statement is true.
(e)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is false.
Explanation of Solution
A carbocation is a carbon atom with three bonds and is deficient in one pair of the electrons, thus, acquiring a positive charge. Therefore, the given statement is false.
(f)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
The carbocation is derived from ethylene by the attack of a proton as depicted in the equation is CH3 CH2 +.
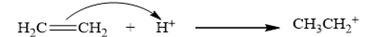
Therefore, the given statement is true.
(g)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
The mechanism of the addition of HX to an alkene is a two-step reaction.
Step 1: The formation of the carbocation. In the first step of the reaction, the proton is added to the double bond, and the carbocation is generated as carbocation intermediate.
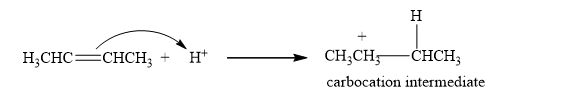
Step 2: The Nucleophilic attack. Then, a halide ion which is a nucleophile attacks the carbocation, forming an additional product.
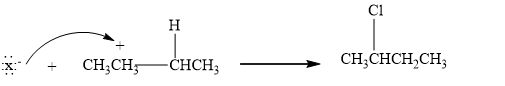
Therefore, the given statement is true.
(h)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
The addition of water is known as hydration. According to Markovnikov's rule, in acid, the catalyzed addition of water to an alkene water molecule is added to the carbon-carbon double bond. Furthermore, in the presence of acid, the alkene is converted to alcohol. Therefore, the given statement is true.
(i)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
The addition of Br2 to a carbon-carbon double bond takes place at room temperature. Thus, if a compound fails to react with Br2, then the compound does not contain a double bond. Therefore, the given statement is true.
(j)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is true.
Explanation of Solution
The addition of H2 is known as reduction. During a reaction with molecular H2, all of the alkenes, are converted to alkanes. Hence, hydrogen is added to the double bond of an alkene. Thus, the given statement is true.
(k)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is false.
Explanation of Solution
Catalytic reduction is the process in which a substance is reduced by hydrogen in the presence of a catalyst. The catalytic reduction of cyclohexene is the addition of H2 across the double bond that is present in the cyclic ring. The resultant product is cyclohexane, not hexane. Thus, the given statement is false.
(l)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is false.
Explanation of Solution
The acid catalyzed hydration of alkene is completed in three steps. The first step includes the addition of a proton (from acid) to the double bond that results in the formation of a carbocation, and hydrogen attaches to one of the doubly bonded carbons. In the second step, an oxygen atom of a water molecule attacks the positively charged carbon, and the water molecule attaches to it, forming an intermediate called an oxonium ion. In the third step, the proton is regenerated again by losing H+ from the oxonium ion, Therefore, in the final product, OH comes from a water molecule, while H comes from the acid used as a catalyst. Thus, the given statement is false.
(m)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is false.
Explanation of Solution
Oxidation is characterized as a gain in oxygen or a loss of hydrogen or a molecule. The alkene on the acid-catalyzed hydration is converted into alcohol. Thus, the conversion of CH2 = CH2 to ethanol is an addition reaction in which a molecule of water is added to the double bond. Thus, there is neither a loss of hydrogen nor a gain of oxygen. Hence, this is an addition reaction and not an oxidation reaction, so, the given statement is false.
(n)
Interpretation:
The given statement is true or false should be determined.
Concept Introduction:
Alkenes or olefins are the unsaturated hydrocarbons. They are organic compounds with one or more carbon-carbon double bonds. Alkenes are more reactive than alkanes. They undergo many characteristic reactions namely Addition reactions, oxidation reactions and combustion reactions.
Answer to Problem 29P
The statement is false.
Explanation of Solution
According to Markovnikov's rule, in the acid-catalyzed hydration of alkene, water is added to the double bond of alkene such that the addition of the H and OH groups occur. Furthermore, it says that H is added to the carbon with more of the hydrogen atoms. The acid catalyzed hydration of 1-butene and 2-butene both result in the formation of 2-butanol. Hence the given statement is false.
Want to see more full solutions like this?
Chapter 12 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forwardI need help with the follloaingarrow_forwardFor a CARS experiment on a Raman band 918 cm-1, if omega1= 1280 nm, calculate the omega2 in wavelength (nm) and the CARS output in wavelength (nm).arrow_forward
- I need help with the following questionarrow_forwardFor CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forward
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forwardCan you help me understand the CBC method on metal bridging by looking at this problem?arrow_forward
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





