
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
12th Edition
ISBN: 9781337915977
Author: Bettelheim
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Question
Chapter 12, Problem 75P
Interpretation Introduction
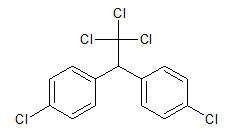
Interpretation:Whether DDT is soluble in water or not should be explained.
Concept Introduction: The molecular formula of DDT is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the Nernst equation to calculate nonstandard cell voltage
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq)
+
2+
2+
3+
Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
☐
x10
μ
Х
5
?
000
日。
Calculating standard reaction free energy from standard reduction...
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction.
Be sure your answer has the correct number of significant digits.
NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq)
kJ
-
☐ x10
x10
olo
18
Ar
Calculating the pH of a weak base titrated with a strong acid
b
An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37.
Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added.
Round your answer to 2 decimal places.
pH = ☐
☑
5
Chapter 12 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
Ch. 12.3 - Prob. 12.1QCCh. 12.3 - Prob. 12.2QCCh. 12.3 - Problem 12-3 Write the IUPAC name for each...Ch. 12.3 - Problem 12-4 Draw structural formulas for the...Ch. 12.3 - Problem 12-5 How many stereoisomers are possible...Ch. 12.5 - Prob. 12.6QCCh. 12.5 - Problem 12-7 Propose a two-step mechanism for the...Ch. 12.5 - Prob. 12.8QCCh. 12.5 - Problem 12-9 Propose a three-step reaction...Ch. 12.5 - Prob. 12.10QC
Ch. 12.8 - Prob. 12.11QCCh. 12 - Prob. 1PCh. 12 - Answer true or false. Both ethylene and acetylene...Ch. 12 - 12-13 What is the difference in structure between...Ch. 12 - There are three compounds with the molecular...Ch. 12 - 12-15 Name and draw structural formulas for all...Ch. 12 - Prob. 6PCh. 12 - Draw a structural formula for at least one...Ch. 12 - Each carbon atom in ethane and in ethylene is...Ch. 12 - Prob. 9PCh. 12 - Prob. 10PCh. 12 - Prob. 11PCh. 12 - 12*22 Draw a structural formula for each compound....Ch. 12 - 12-23 Draw a structural formula for each compound....Ch. 12 - Prob. 14PCh. 12 - 12-25 Write the IUPAC name for each unsaturated...Ch. 12 - Explain why each name is incorrect and then write...Ch. 12 - 12-27 Explain why each name is incorrect and then...Ch. 12 - Prob. 18PCh. 12 - 12-29 Which of these alkenes show cis-trans...Ch. 12 - 12-30 Which of these alkenes shows cis-trans...Ch. 12 - 12-31 Cyclodecene exists as both cis and trans...Ch. 12 - Arachidonic acid is a naturally occurring C„o...Ch. 12 - Prob. 23PCh. 12 - If you examine the structural formulas for the...Ch. 12 - 12*35 For each molecule that shows eis-trans...Ch. 12 - Name and draw structural formulas for all...Ch. 12 - /3-Ocimene, a triene found in the fragrance of...Ch. 12 - Answer true or false. Alkenes and alkynes are...Ch. 12 - Prob. 29PCh. 12 - 12-40 Define alkene addition reaction. Write an...Ch. 12 - Prob. 31PCh. 12 - 12-42 Complete these equations.Ch. 12 - Draw structural formulas for all possible...Ch. 12 - Prob. 34PCh. 12 - 12-45 Draw a structural formula for the product of...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - 12-47 Draw a structural formula for an alkene with...Ch. 12 - Draw a structural formula for an alkene with the...Ch. 12 - Prob. 39PCh. 12 - 12-50 Draw the structural formula of an alkene...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Following is the structural formula of...Ch. 12 - Propose an explanation for the following...Ch. 12 - There are nine alkenes with the molecular formula...Ch. 12 - Prob. 46PCh. 12 - 12-57 Hydrocarbon A, Cf,Hs, reacts with 2 moles of...Ch. 12 - 12-58 Show how to convert ethylene to these...Ch. 12 - 12-59 Show how to convert 1-butene to these...Ch. 12 - Prob. 50PCh. 12 - 12-61 (Chemical Connections 12A) What is one...Ch. 12 - Prob. 52PCh. 12 - Prob. 53PCh. 12 - 12-64 (Chemical Connections 120 What is the...Ch. 12 - (Chemical Connections 120 Assume that 1 X IO-12 g...Ch. 12 - Prob. 56PCh. 12 - Prob. 57PCh. 12 - Prob. 58PCh. 12 - Prob. 59PCh. 12 - Prob. 60PCh. 12 - Prob. 61PCh. 12 - Suppose you have unlabeled bottles of benzene and...Ch. 12 - Prob. 63PCh. 12 - Prob. 64PCh. 12 - Answer true or false. (a) Phenols and alcohols...Ch. 12 - Prob. 66PCh. 12 - Prob. 67PCh. 12 - Prob. 68PCh. 12 - Prob. 69PCh. 12 - 12-67 (Chemical Connections 12D ) In which isomer...Ch. 12 - Prob. 71PCh. 12 - Prob. 72PCh. 12 - Prob. 73PCh. 12 - Prob. 74PCh. 12 - Prob. 75PCh. 12 - Prob. 76PCh. 12 - Prob. 77PCh. 12 - Prob. 78PCh. 12 - Prob. 79PCh. 12 - Prob. 80PCh. 12 - Prob. 81PCh. 12 - Prob. 82PCh. 12 - Prob. 83PCh. 12 - Prob. 84PCh. 12 - Prob. 85PCh. 12 - Prob. 86PCh. 12 - Propose a structural formula for the product!s)...Ch. 12 - Prob. 88PCh. 12 - Draw the structural formula of an alkene that...Ch. 12 - 12-77 Show how to convert cyclopentene into these...Ch. 12 - Prob. 91PCh. 12 - Prob. 92PCh. 12 - Prob. 93PCh. 12 - Prob. 94PCh. 12 - Prob. 95PCh. 12 - In omega-3 fatty adds, the last carbon of the last...Ch. 12 - Prob. 97PCh. 12 - Prob. 98P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forwardQUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forward
- Question: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forward
- The following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forward
- Briefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY