
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 12.49E
Limonene, which is present in citrus peelings, has a very pleasant lemonlike fragrance. However, it is not classified as an
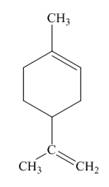
limonene
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Explain the inductive effect (+I and -I) in benzene derivatives.
The inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?
19.57 Using one of the reactions in this chapter, give the correct starting material (A-L)
needed to produce each structure (a-f). Name the type of reaction used.
(b)
ہ مرد
(d)
HO
(c)
དང་ ་་ཡིན་ད་དང་
(f)
HO
Br
B
D
of
oli
H
J
Br
K
C
人
↑
Chapter 12 Solutions
Chemistry for Today: General, Organic, and Biochemistry
Ch. 12 - Prob. 12.1ECh. 12 - Define the terms alkene, alkyne, and aromatic...Ch. 12 - Select those compounds that can be correctly...Ch. 12 - Prob. 12.4ECh. 12 - Give the IUPAC name for the following compounds:...Ch. 12 - Prob. 12.6ECh. 12 - Prob. 12.7ECh. 12 - Prob. 12.8ECh. 12 - Prob. 12.9ECh. 12 - Prob. 12.10E
Ch. 12 - Prob. 12.11ECh. 12 - Prob. 12.12ECh. 12 - What type of hybridized orbital is present on...Ch. 12 - What type of orbital overlaps to form a pi bond in...Ch. 12 - Prob. 12.15ECh. 12 - Explain the difference between geometric and...Ch. 12 - Draw structural formulas and give IUPAC names for...Ch. 12 - Prob. 12.18ECh. 12 - Which of the following alkenes can exist as...Ch. 12 - Draw structural formulas for the following:...Ch. 12 - Prob. 12.21ECh. 12 - In what ways are the physical properties of...Ch. 12 - Prob. 12.23ECh. 12 - Prob. 12.24ECh. 12 - Complete the following reactions. Where more than...Ch. 12 - Prob. 12.26ECh. 12 - Prob. 12.27ECh. 12 - What reagents would you use to prepare each of the...Ch. 12 - What is an important commercial application of...Ch. 12 - Prob. 12.30ECh. 12 - Terpin hydrate is used medicinally as an...Ch. 12 - Prob. 12.32ECh. 12 - Prob. 12.33ECh. 12 - Prob. 12.34ECh. 12 - Prob. 12.35ECh. 12 - Much of todays plumbing in newly built homes is...Ch. 12 - Prob. 12.37ECh. 12 - What type of hybridized orbital is present on...Ch. 12 - How many sigma bonds and how many pi bonds make up...Ch. 12 - Prob. 12.40ECh. 12 - Explain why geometric isomerism is not possible in...Ch. 12 - Give the common name and major uses of the...Ch. 12 - Describe the physical and chemical properties of...Ch. 12 - Prob. 12.44ECh. 12 - Prob. 12.45ECh. 12 - Prob. 12.46ECh. 12 - Prob. 12.47ECh. 12 - Prob. 12.48ECh. 12 - Limonene, which is present in citrus peelings, has...Ch. 12 - A disubstituted cycloalkane such as a exhibits...Ch. 12 - Prob. 12.51ECh. 12 - Prob. 12.52ECh. 12 - Give an IUPAC name for the following as...Ch. 12 - Give an IUPAC name for the following as...Ch. 12 - Name the following compounds, using the prefixed...Ch. 12 - Name the following compounds, using the prefixed...Ch. 12 - Name the following by numbering the benzene ring....Ch. 12 - Name the following by numbering the benzene ring....Ch. 12 - Prob. 12.59ECh. 12 - Write structural formulas for the following:...Ch. 12 - Prob. 12.61ECh. 12 - Prob. 12.62ECh. 12 - Prob. 12.63ECh. 12 - Prob. 12.64ECh. 12 - Prob. 12.65ECh. 12 - Prob. 12.66ECh. 12 - Prob. 12.67ECh. 12 - Prob. 12.68ECh. 12 - Prob. 12.69ECh. 12 - Prob. 12.70ECh. 12 - Prob. 12.71ECh. 12 - Prob. 12.72ECh. 12 - Prob. 12.73ECh. 12 - Prob. 12.74ECh. 12 - Prob. 12.75ECh. 12 - Prob. 12.76ECh. 12 - Prob. 12.77ECh. 12 - Prob. 12.78ECh. 12 - Prob. 12.79ECh. 12 - Prob. 12.80ECh. 12 - Prob. 12.81ECh. 12 - Prob. 12.82ECh. 12 - Prob. 12.83ECh. 12 - The compound CH2=CHCH2CH2CH3 is an example of: a....Ch. 12 - The correct structural for ethyne is: a. HC=CH b....Ch. 12 - Prob. 12.86E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY