
Concept explainers
(a)
Interpretation:
Whether the
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 12.18E
The alkene
Explanation of Solution
The alkene is
A compound shows cis-trans isomerism when the substituent groups on the carbon atoms that are connected via double bond are different. The alkene has the same substituted groups on a carbon atom that is on the right-hand side of the double bond. Therefore; it will not have cis-trans isomerism.
The alkene
(b)
Interpretation:
Whether the alkene
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. This phenomenon is called isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 12.18E
The alkene
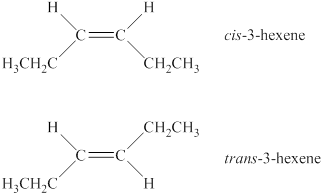
Explanation of Solution
The alkene is
A compound shows cis-trans isomerism when the substituents groups on the carbon atoms that are connected via double bond are different. The alkene has two different substituted groups on carbon atoms that are connected via a double bond. Therefore, it will show cis-trans isomerism. In the cis isomer, same substituted groups are on the same side of a ring or double bonds, whereas in the trans isomer same substituted groups are on a different side of a ring or double bond. Therefore, the structures of cis-trans isomers of the alkene are shown below.

Figure 1
The alkene
(c)
Interpretation:
Whether the alkene
Concept introduction:
Those compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 12.18E
The alkene
Explanation of Solution
The structure of the alkene
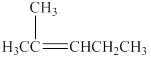
Figure 2
A compound shows cis-trans isomerism when the substituents groups on the carbon atoms that are connected via double bond are different. The alkene has the same substituted groups on a carbon atom that is on the left-hand side of the double bond. Therefore, it will not have cis-trans isomerism.
The alkene
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- X Draw the major products of the elimination reaction below. If elimination would not occur at a significant rate, check the box under the drawing area instead. ది www. Cl + OH Elimination will not occur at a significant rate. Click and drag to start drawing a structure.arrow_forwardNonearrow_forward1A H 2A Li Be Use the References to access important values if needed for this question. 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 5B 6B 7B 8B-1B 2B Al Si P 1B 2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha ****** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. Use the smallest integers possible. cation anion + + Shell 1: 2 Shell 2: 8 Shell 3: 1 Shell 1 : 2 Shell 2 : 6 Shell 1 : 2 Shell 2: 8 Shell 1: 2 Shell 2: 8arrow_forward
- Nonearrow_forwardIV. Show the detailed synthesis strategy for the following compounds. a. CH3CH2CH2CH2Br CH3CH2CCH2CH2CH3arrow_forwardDo the electrons on the OH participate in resonance with the ring through a p orbital? How many pi electrons are in the ring, 4 (from the two double bonds) or 6 (including the electrons on the O)?arrow_forward
- Predict and draw the product of the following organic reaction:arrow_forwardNonearrow_forwardRedraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately represent the direction of the bonds to ring substituents. Cl. Br Click and drag to start drawing a structure. : ☐ ☑ Parrow_forward
- K m Choose the best reagents to complete the following reaction. L ZI 0 Problem 4 of 11 A 1. NaOH 2. CH3CH2CH2NH2 1. HCI B OH 2. CH3CH2CH2NH2 DII F1 F2 F3 F4 F5 A F6 C CH3CH2CH2NH2 1. SOCl2 D 2. CH3CH2CH2NH2 1. CH3CH2CH2NH2 E 2. SOCl2 Done PrtScn Home End FA FQ 510 * PgUp M Submit PgDn F11arrow_forwardNonearrow_forwardPlease provide a mechanism of synthesis 1,4-diaminobenzene, start from a benzene ring.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





