
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.25E
Complete the following reactions. Where more than one product is possible, show only the one expected according to Markovnikov’s rule.
a. 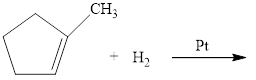
b.
c. 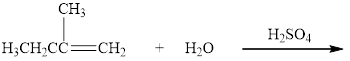
d. 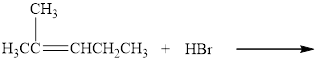
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the curved-arrow mechanism with the drawings of the molecules, not
just abbreviations.
-NO₂
Sn, HCl (aq)
E
D
H
(CH3CO)₂O
-NH2
CH3
What is/are the product(s) of the following reaction? Select all that apply.
* HI
A
B
C
OD
OH
A
B
OH
D
C
In the image, the light blue sphere represents a mole of hydrogen atoms, the purple or teal spheres represent a mole of a conjugate base. A light blue sphere by itself is H+.
Assuming there is 2.00 L of solution, answer the following:
The Ka of the left & right solution is?
The pH of the left & right solution is?
The acid on the left & right is what kind of acid?
Chapter 12 Solutions
Chemistry for Today: General, Organic, and Biochemistry
Ch. 12 - Prob. 12.1ECh. 12 - Define the terms alkene, alkyne, and aromatic...Ch. 12 - Select those compounds that can be correctly...Ch. 12 - Prob. 12.4ECh. 12 - Give the IUPAC name for the following compounds:...Ch. 12 - Prob. 12.6ECh. 12 - Prob. 12.7ECh. 12 - Prob. 12.8ECh. 12 - Prob. 12.9ECh. 12 - Prob. 12.10E
Ch. 12 - Prob. 12.11ECh. 12 - Prob. 12.12ECh. 12 - What type of hybridized orbital is present on...Ch. 12 - What type of orbital overlaps to form a pi bond in...Ch. 12 - Prob. 12.15ECh. 12 - Explain the difference between geometric and...Ch. 12 - Draw structural formulas and give IUPAC names for...Ch. 12 - Prob. 12.18ECh. 12 - Which of the following alkenes can exist as...Ch. 12 - Draw structural formulas for the following:...Ch. 12 - Prob. 12.21ECh. 12 - In what ways are the physical properties of...Ch. 12 - Prob. 12.23ECh. 12 - Prob. 12.24ECh. 12 - Complete the following reactions. Where more than...Ch. 12 - Prob. 12.26ECh. 12 - Prob. 12.27ECh. 12 - What reagents would you use to prepare each of the...Ch. 12 - What is an important commercial application of...Ch. 12 - Prob. 12.30ECh. 12 - Terpin hydrate is used medicinally as an...Ch. 12 - Prob. 12.32ECh. 12 - Prob. 12.33ECh. 12 - Prob. 12.34ECh. 12 - Prob. 12.35ECh. 12 - Much of todays plumbing in newly built homes is...Ch. 12 - Prob. 12.37ECh. 12 - What type of hybridized orbital is present on...Ch. 12 - How many sigma bonds and how many pi bonds make up...Ch. 12 - Prob. 12.40ECh. 12 - Explain why geometric isomerism is not possible in...Ch. 12 - Give the common name and major uses of the...Ch. 12 - Describe the physical and chemical properties of...Ch. 12 - Prob. 12.44ECh. 12 - Prob. 12.45ECh. 12 - Prob. 12.46ECh. 12 - Prob. 12.47ECh. 12 - Prob. 12.48ECh. 12 - Limonene, which is present in citrus peelings, has...Ch. 12 - A disubstituted cycloalkane such as a exhibits...Ch. 12 - Prob. 12.51ECh. 12 - Prob. 12.52ECh. 12 - Give an IUPAC name for the following as...Ch. 12 - Give an IUPAC name for the following as...Ch. 12 - Name the following compounds, using the prefixed...Ch. 12 - Name the following compounds, using the prefixed...Ch. 12 - Name the following by numbering the benzene ring....Ch. 12 - Name the following by numbering the benzene ring....Ch. 12 - Prob. 12.59ECh. 12 - Write structural formulas for the following:...Ch. 12 - Prob. 12.61ECh. 12 - Prob. 12.62ECh. 12 - Prob. 12.63ECh. 12 - Prob. 12.64ECh. 12 - Prob. 12.65ECh. 12 - Prob. 12.66ECh. 12 - Prob. 12.67ECh. 12 - Prob. 12.68ECh. 12 - Prob. 12.69ECh. 12 - Prob. 12.70ECh. 12 - Prob. 12.71ECh. 12 - Prob. 12.72ECh. 12 - Prob. 12.73ECh. 12 - Prob. 12.74ECh. 12 - Prob. 12.75ECh. 12 - Prob. 12.76ECh. 12 - Prob. 12.77ECh. 12 - Prob. 12.78ECh. 12 - Prob. 12.79ECh. 12 - Prob. 12.80ECh. 12 - Prob. 12.81ECh. 12 - Prob. 12.82ECh. 12 - Prob. 12.83ECh. 12 - The compound CH2=CHCH2CH2CH3 is an example of: a....Ch. 12 - The correct structural for ethyne is: a. HC=CH b....Ch. 12 - Prob. 12.86E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What spectral features allow you to differentiate the product from the starting material? Use four separate paragraphs for each set of comparisons. You should have one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of functional group changes.arrow_forwardQuestion 6 What is the major product of the following Diels-Alder reaction? ? Aldy by day of A. H о B. C. D. E. OB OD Oc OE OAarrow_forwardNonearrow_forward
- In the solid state, oxalic acid occurs as a dihydrate with the formula H2C2O4 C+2H2O. Use this formula to calculate the formula weight of oxalic acid. Use the calculated formula weight and the number of moles (0.00504mol) of oxalic acid in each titrated unknown sample recorded in Table 6.4 to calculate the number of grams of pure oxalic acid dihydrate contained in each titrated unknown sample.arrow_forward1. Consider a pair of elements with 2p and 4p valence orbitals (e.g., N and Se). Draw their (2p and 4p AO's) radial probability plots, and sketch their angular profiles. Then, consider these orbitals from the two atoms forming a homonuclear л-bond. Which element would have a stronger bond, and why? (4 points)arrow_forwardWrite the reaction and show the mechanism of the reaction. Include the mechanism for formation of the NO2+ 2. Explain, using resonance structures, why the meta isomer is formed. Draw possible resonance structures for ortho, meta and para.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY