
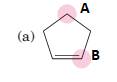
(a)
Interpretation:
To predict bond angles about each highlighted carbon.
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
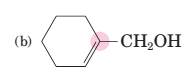
(b)
Interpretation:
To predict bond angles about each highlighted carbon.
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
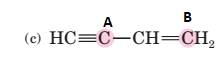
(c)
Interpretation:
To predict bond angles about each highlighted carbon.
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles. |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
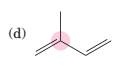
(d)
Interpretation:
To predict bond angles about each highlighted carbon.
Concept Introduction:
VSEPR predicts the geometry and the bond angles for the molecules according to the theory, the structure is predicted by the lone pairs and bond pairs present in the bond formation.
Electron pair = Lone pair + Bond pair.
| Electron pair | Hybridization | Geometry | Bond angles. |
| 2 | sp | Linear | 180° |
| 3 | sp2 | Trigonal planar | 120° |
| 4 | sp3 | Tetrahedra or square planar | 109.5° or 90° |
| 5 | sp3 d | Trigonal bipyramidal | 120° and 90° |
| 6 | sp3 d2 | Octahedral | 90° |
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- 1. For the four structures provided, Please answer the following questions in the table below. a. Please draw π molecular orbital diagram (use the polygon-and-circle method if appropriate) and fill electrons in each molecular orbital b. Please indicate the number of π electrons c. Please indicate if each molecule provided is anti-aromatic, aromatic, or non- aromatic TT MO diagram Number of π e- Aromaticity Evaluation (X choose one) Non-aromatic Aromatic Anti-aromatic || ||| + IVarrow_forward1.3 grams of pottasium iodide is placed in 100 mL of o.11 mol/L lead nitrate solution. At room temperature, lead iodide has a Ksp of 4.4x10^-9. How many moles of precipitate will form?arrow_forwardQ3: Circle the molecules that are optically active: ДДДДarrow_forward
- 6. How many peaks would be observed for each of the circled protons in the compounds below? 8 pts CH3 CH3 ΤΙ A. H3C-C-C-CH3 I (₁₁ +1)= 7 H CI B. H3C-C-CI H (3+1)=4 H LIH)=2 C. (CH3CH2-C-OH H D. CH3arrow_forwardNonearrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? H Br H Br (S) CH3 (R) CH3 H3C (S) H3C H Br Br H A C enantiomers H Br H Br (R) CH3 H3C (R) (S) CH3 H3C H Br Br H B D identicalarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





