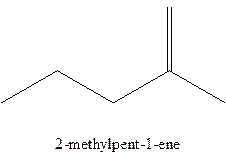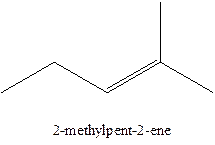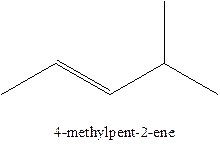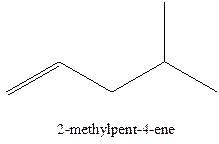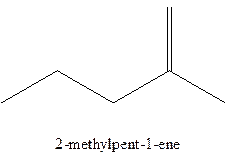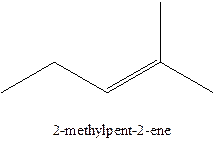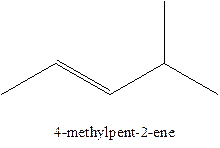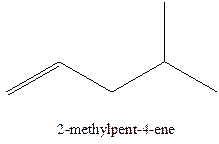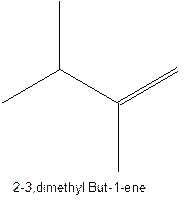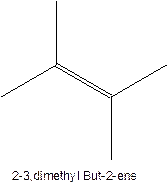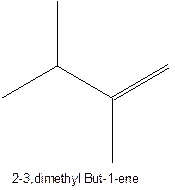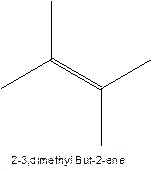Concept explainers
(a)
Interpretation:
To name and draw structural formula for
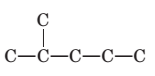
Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
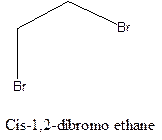
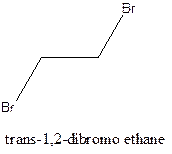
Answer to Problem 12.16P
The alkenes with the molecular formula
Explanation of Solution
The longest chain for the given skeleton has 5 carbons therefore the root name prop- is used here. The alkenes with the molecular formula
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
(b)
Interpretation:
To name and draw structural formula for alkenes with the molecular formula
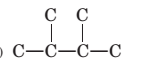
Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
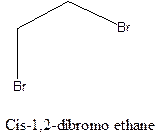
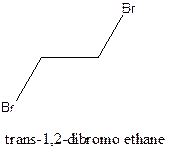
Answer to Problem 12.16P
The alkenes with the molecular formula
Explanation of Solution
The longest chain for the given skeleton has 4 carbons therefore the root name but- is used here. The alkenes with the molecular formula
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
(c)
Interpretation:
To name and draw structural formula for alkenes with the molecular formula
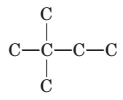
Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
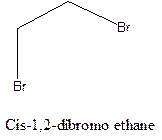
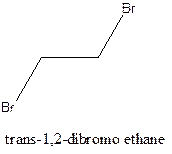
Answer to Problem 12.16P
The alkene with the molecular formula
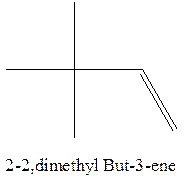
Explanation of Solution
The longest chain for the given skeleton has 4 carbons therefore the root name but- is used here. The alkene with the molecular formula
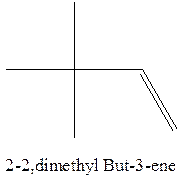
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
(c)
Interpretation:
To name and draw structural formula for alkenes with the molecular formula

Concept Introduction:
Isomers are the compounds that have same molecular formula but different structural formula.
Cis-trans isomerism arises in the compounds when there is a difference in the orientation of the two same groups.
When two same groups are along the same side of the C-C bond it is called as cis isomer, but when the same groups lie opposite to each other along the C-C bond. It is said to be trans isomer.
Example- Cis-1,2-dibromo ethane and trans-1,2, dibromo ethane are cis-trans isomers.
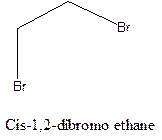
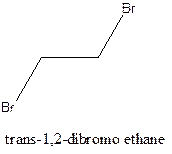
Answer to Problem 12.16P
The alkene with the molecular formula
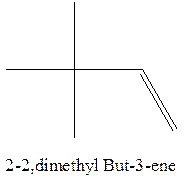
Explanation of Solution
The longest chain for the given skeleton has 4 carbons therefore the root name but- is used here. The alkene with the molecular formula
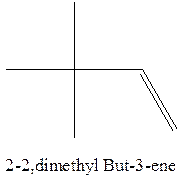
In this isomer, it contains 6 carbons and 12 hydrogen atoms, thus the molecular formula is
Want to see more full solutions like this?
Chapter 12 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning