
Concept explainers
(a)
Interpretation:
The given statement is true or false should be determined.
The IUPAC name of an alkene is derived from the name of the longest carbon chain that contains the carbon-carbon double bond.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism. If same groups are present on the same side, then the isomer is called as cis-isomer and the same groups are present on different side the isomer is called as trans-isomer.
Answer to Problem 12.21P
The given statement is true.
Explanation of Solution
As per general rules of
(b)
Interpretation:
The given statement is true or false should be determined.
The IUPAC name of
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side, then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
IUPAC name of
Therefore, the given statement is false.
(c)
Interpretation:
The given statement is true or false should be determined.
2-Methyl-2-butene shows cis-trans isomerism.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side, then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
2-Methyl-2-butene contains two methyl groups present on carbon atom of carbon-carbon double bond other carbon contains also methyl group.
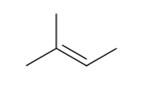
To get cis and trans isomers, two different groups should be present on the carbon atoms of the carbons with double bond.
Thus, the statement is false.
(d)
Interpretation:
The given statement is true or false should be determined.
1,2-dimethylcyclohexene shows cis-trans isomerism.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
Following are the structures of the isomers of 1,2-dimethylcyclohexane. Form this, we can see that all four atoms of carbon-carbon double bond are lying in same plane.
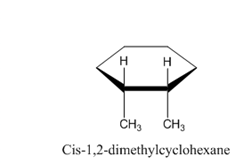
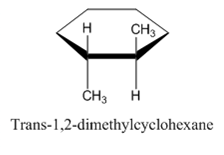
Therefore, the given statement is false.
(e)
Interpretation:
The given statement is true or false should be determined.
The IUPAC name of
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is true.
Explanation of Solution
The IUPAC name of
(f)
Interpretation:
The given statement is true or false should be determined.
1,3-Butadiene has two carbon-carbon double bonds and 22 =4 stereoisomers are possible for it.
Concept Introduction:
The IUPAC name is the set of rules that is created and applied by the International Union of pure and Applied Chemistry to generate systematic proper name of chemical compounds. This nomenclature is used to describe and identify the type and position of functional groups, side chains, double or triple bonds etc. in alphabetical order to provide systematic name to compound.
Alkenes are the compounds with very weak London dispersion forces and are nonpolar. They contain same skeleton structure and physical properties like alkanes but liquid at room temperature.
Cis-trans stereoisomerisms are seen in alkenes compounds. Due to presence of two different groups bonded to carbon of carbon-carbon double bond, restricted action occurs in it and results into cis-trans isomerism.
If same groups are present on the same side, then the isomer is called as cis- isomer and the same groups are present on different side the isomer is called as trans- isomer.
Answer to Problem 12.21P
The given statement is false.
Explanation of Solution
Following is the structure of 1,3-Butadiene. There are no stereocenters are present in the structure of 1,3-Butadiene. There should be presence of different groups for isomerism.
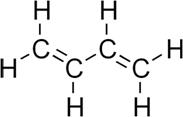
Therefore, the given statement is false.
Want to see more full solutions like this?
Chapter 12 Solutions
Bundle: Introduction to General, Organic and Biochemistry, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
- You are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forwardPredict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




