
Concept explainers
(a)
Interpretation:
The molecular formula for the given hydrocarbon has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(a)
Answer to Problem 12.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of cycloalkane is 1,2-dimethylcyclopentane. From the name it is understood that the parent cycloalkane is five carbon cyclic ring which is cyclopentane. The substituents present on the cyclopentane ring are two methyl groups, each at first and second carbon. Therefore, the line-angle structural formula for 1,2-dimethylcyclopentane can be drawn as shown below,
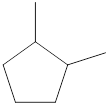
The above structure is a line-angle structural formula representation. This structure has a total of five intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be seven.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given hydrocarbon is found to be
The molecular formula for the given hydrocarbon is found as
(b)
Interpretation:
The molecular formula for the given hydrocarbon has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(b)
Answer to Problem 12.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of cycloalkane is 1,3-dimethylcyclopentane. From the name it is understood that the parent cycloalkane is five carbon cyclic ring which is cyclopentane. The substituent present on the cyclopentane ring are two methyl groups, each at first and third carbon. Therefore, the line-angle structural formula for 1,3-dimethylcyclopentane can be drawn as shown below,

The above structure is a line-angle structural formula representation. This structure has a total of five intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be seven.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given hydrocarbon is found to be
The molecular formula for the given hydrocarbon is found as
(c)
Interpretation:
Molecular formula for the given hydrocarbon has to be determined.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Molecular formula of the
(c)
Answer to Problem 12.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of alkane is 2,2-dimethylpentane. From the name it is understood that the parent alkane is five carbon chain which is pentane. The substituent present on the hexane are two methyl groups, on the second carbon atom. Therefore, the line-angle structural formula for 2,2-dimethylpentane can be drawn as shown below,
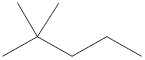
In line-angle structural representation of molecule, carbon atom is present where two lines meet and at the end of the lines. In the above structure there are three points where the lines meet and four end points. Therefore, the total number of carbon atoms present in the given structure is 7.
To determine the molecular formula of alkane, the value of “n” has to be substituted as 7 in the general formula of alkane.
Therefore, the molecular formula of the given hydrocarbon is determined as
The molecular formula of the hydrocarbon was determined as
(d)
Interpretation:
Molecular formula for the given hydrocarbon has to be determined.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Molecular formula of the alkanes can be found by using the general formula
(d)
Answer to Problem 12.108EP
The molecular formula for the given hydrocarbon is
Explanation of Solution
Given name of alkane is 2,3-dimethylpentane. From the name it is understood that the parent alkane is five carbon chain which is pentane. The substituent present on the hexane are two methyl groups, each at second and third carbon. Therefore, the line-angle structural formula for 2,3-dimethylpentane can be drawn as shown below,
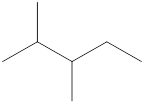
In line-angle structural representation of molecule, carbon atom is present where two lines meet and at the end of the lines. In the above structure there are three points where the lines meet and four end points. Therefore, the total number of carbon atoms present in the given structure is 7.
To determine the molecular formula of alkane, the value of “n” has to be substituted as 7 in the general formula of alkane.
Therefore, the molecular formula of the given hydrocarbon is determined as
The molecular formula of the given hydrocarbon was determined as
Want to see more full solutions like this?
Chapter 12 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Select all of the following that changes in the MC1R gene can lead to: Changes in spots/stripes in lizards, changes in coat coloration in mice, ectopic ear formation in Siberian hamsters, and red hair in humansarrow_forwardPleiotropic genes are genes that (blank) Cause a swapping of organs/structures, are the result of duplicated sets of chromosomes, never produce protein products, and have more than one purpose/functionarrow_forwardA loss of function mutation in Pitx1 enhancers can cause (blank) Removal of Pitx1 exons and growth of ectopic hindlimbs, growth of extra ectopic forelimbs, loss of forelimb specification and development, and loss of hindlimb specification and developmentarrow_forward
- Hox1a most likely contributes to (blank) patterning in the developing embryo? Ventral, posterior, limb or anteriorarrow_forwardSelect all of the following that can help establish Hox gene expression boundaries (things that affect Hox and not things that Hox affects). Retinoic acid, anterior/posterior axis, fibroblast growth factors, vagal neural crest, and enhancersarrow_forwardEctopic expression of Hox often results in (blank) phenotypes. (Blank) transformations are characterized by the replacement of one body part/structure with another. Hoxeotic, homealoneotic, joexotic, or homeoticarrow_forward
- What's the difference when drawing omega-6 and omega-3?arrow_forward. Consider a base substitution mutation that occurred in a DNA sequence that resulted in a change in the encoded protein from the amino acid glutamic acid to aspartic acid. Normally the glutamic acid amino acid is located on the outside of the soluble protein but not near an active site. O-H¨ A. What type of mutation occurred? O-H B. What 2 types of chemical bonds are found in the R-groups of each amino acid? The R groups are shaded. CH2 CH2 CH2 H2N-C-COOH H2N-C-COOH 1 H Glutamic acid H Aspartic acid C. What 2 types of bonds could each R-group of each of these amino acids form with other molecules? D. Consider the chemical properties of the two amino acids and the location of the amino acid in the protein. Explain what effect this mutation will have on this protein's function and why.arrow_forwardengineered constructs that consist of hollow fibers are acting as synthetic capillaries, around which cells have been loaded. The cellular space around a single fiber can be modeled as if it were a Krogh tissue cylinder. Each fiber has an outside “capillary” radius of 100 µm and the “tissue” radius can be taken as 200 µm. The following values apply to the device:R0 = 20 µM/secaO2 = 1.35 µM/mmHgDO2,T = 1.67 x 10-5 cm2/secPO2,m = 4 x 10-3 cm/secInstead of blood inside the fibers, the oxygen transport and tissue consumption are being investigated by usingan aqueous solution saturated with pure oxygen. As a result, there is no mass transfer resistance in the synthetic“capillary”, only that due to the membrane itself. Rather than accounting for pO2 variations along the length ofthe fiber, use an average value in the “capillary” of 130 mmHg.Is the tissue fully oxygenated?arrow_forward
- Molecular Biology Please help with question. thank you You are studying the expression of the lac operon. You have isolated mutants as described below. In the presence of glucose, explain/describe what would happen, for each mutant, to the expression of the lac operon when you add lactose AND what would happen when the bacteria has used up all of the lactose (if the mutant is able to use lactose).5. Mutations in the lac operator that strengthen the binding of the lac repressor 200 fold 6. Mutations in the promoter that prevent binding of RNA polymerase 7. Mutations in CRP/CAP protein that prevent binding of cAMP8. Mutations in sigma factor that prevent binding of sigma to core RNA polymerasearrow_forwardMolecular Biology Please help and there is an attached image. Thank you. A bacteria has a gene whose protein/enzyme product is involved with the synthesis of a lipid necessary for the synthesis of the cell membrane. Expression of this gene requires the binding of a protein (called ACT) to a control sequence (called INC) next to the promoter. A. Is the expression/regulation of this gene an example of induction or repression?Please explain:B. Is this expression/regulation an example of positive or negative control?C. When the lipid is supplied in the media, the expression of the enzyme is turned off.Describe one likely mechanism for how this “turn off” is accomplished.arrow_forwardMolecular Biology Please help. Thank you. Discuss/define the following:(a) poly A polymerase (b) trans-splicing (c) operonarrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning





