
Concept explainers
(a)
Interpretation: The alkyl halide that is used to form the given
Concept introduction: The reaction of
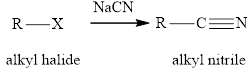
The hydrolysis reaction of alkyl nitrile in presence of acid is written as:
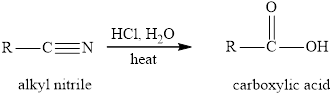
(b)
Interpretation: The alkyl halide that is used to form the given carboxylic acid has to be identified.
Concept introduction: The reaction of alkyl halides with sodium cyanide gives alkyl cyanides or nitriles. After the reaction there is increase incarbon atoms of the alkyl chain of alkyl halide. The nitriles obtained undergo hydrolysis reaction in presence of acid and gives carboxylic acid. The general reaction of alkyl halides and sodum cyanide is written as:
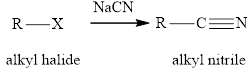
The hydrolysis reaction of alkyl nitrile in presence of acid is written as:
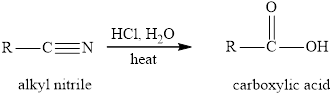
(c)
Interpretation: The alkyl halide that is used to form the given carboxylic acid has to be identified.
Concept introduction: The reaction of alkyl halides with sodium cyanide gives alkyl cyanides or nitriles. After the reaction there is increase incarbon atoms of the alkyl chain of alkyl halide. The nitriles obtained undergo hydrolysis reaction in presence of acid and gives carboxylic acid. The general reaction of alkyl halides and sodum cyanide is written as:
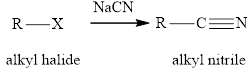
The hydrolysis reaction of alkyl nitrile in presence of acid is written as:
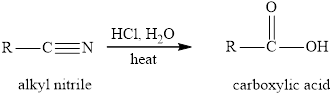
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




