
(a)
Interpretation:
The expected products obtained from the given reaction have to be predicted.
Concept introduction:
Esterification reaction and intermolecular esterification reactions:
General scheme for esterification reaction:
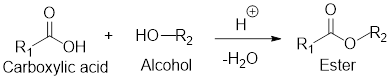
If the carboxylic acid and alcohol
Example for intermolecular esterification reaction:
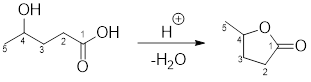
(b)
Interpretation:
The expected products obtained from the given reaction have to be predicted.
Concept introduction:
Transesterification and intermolecular transesterification reactions:
In the presence of an acid, ester and alcohol forms another new ester which is known as transester and a new alcohol will also be formed. This type of reaction is called transesterification reaction.
General scheme for transesterification reaction:
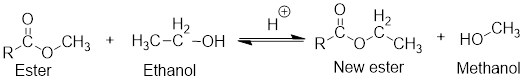
If the ester and alcohol are present within the same compound, then cyclic ester is formed which is known as lactone and the reaction is known as intermolecular transesterification reaction.
Example for intermolecular transesterification reaction:
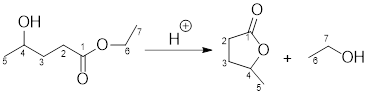
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
- no Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forwardno Ai walkthroughsarrow_forward
- 3. Synthesize the following synthon from the indicated starting material. i HO.arrow_forwardIdentifying the stereochemistry of natural Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule H O-C-CH2 H3N. HN N H C=O common name (not the IUPAC name) NH3 ☐ H3N H ☐ CH2 Xarrow_forward> Draw the structure of alanine at pH 1.2. Click and drag to start drawing a structure.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

