
Concept explainers
(a)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
Transesterification: Transesterification reaction is an esterification reaction of ester react with excess of alcohol in the presence of either acid or base catalyst to form a new ester. The formation of one type of ester can be transformed in to other form of esters is called esterification when reaction moves forward when we use excess of an alcohol.

(b)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Ester reaction with sodium hydroxide which goves the sodium salt and alcohol.
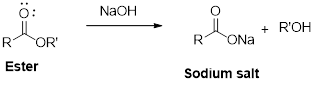
It is an example of saponification reaction.
(c)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Amination reaction: Amination is the process by which an
(d)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Ester reaction with sodium hydroxide which gives the sodium salt and alcohol this sodium salt further reaction with dil. Hydrochloric acid gives acid.

It is an example of saponification reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
- Please explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forward
- (a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forwardPlease helparrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

