
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.93AP
Answer the following questions for the cycloalkane depicted in the given ball-and-stick model.
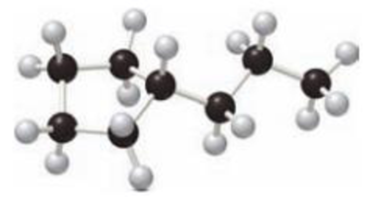
- a. Give the IUPAC name.
- b. Draw one constitutional isomer.
- c. Predict the solubility in water.
- d. Predict the solubility in an organic solvent.
- e. Draw a skeletal structure.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Determine whether cis-trans isomerism is possible for each of the following
cycloalkanes. If so, then draw structural formulas for the cis and trans isomers..
a. Methylcyclohexane
b. 1,1-Dimethylcyclohexane
c. 1,3-Dimethylcyclobutane
d. 1-Ethyl-2-methylcyclobutane
1. Provide the IUPAC name.
a.
b.
H3CO.
H₂N
D
O₂N
F
NO2
IUPAC name
IUPAC name
1. In dash-wedge-line structure, the dashes represent the: *
A. bonds in the plane of the page.
B. bonds away from observer.
C. bonds towards observer.
D. bonds in the plane of the paper.
2. Which of the following is an ACYCLIC SATURATED hydrocarbon? *
A. 1-Ethyl-3-methylcyclopentane
B. 3-Ethyl-3-methylpentane
C. 1,3,5,7-Octatetraene
D. 5-Methyl-1,3-cyclopentadiene
3. Which of the following is a CYCLIC SATURATED hydrocarbon? *
A. 3-Ethyl-4-methylcyclohexene
B. 3,5-Diethyloctane
C. 2,2-Dimethyl-3-heptyne
D. 3-Butyl-1,1-dimethyl-5-propylcyclohexane
Chapter 10 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 10.1 - Prob. 10.1PCh. 10.2 - Fill in all Hs and lone pairs in each compound. a....Ch. 10.3 - Convert each compound to a condensed formula.Ch. 10.3 - Convert each condensed formula to a complete...Ch. 10.3 - Convert each skeletal structure to a complete...Ch. 10.3 - How many Hs are bonded to each indicated carbon...Ch. 10.4 - Identify the functional groups in each compound....Ch. 10.4 - For each compound: [1] Identify the functional...Ch. 10.4 - Prob. 10.9PCh. 10.4 - Prob. 10.10P
Ch. 10.4 - Convert the ball-and-stick model of the local...Ch. 10.5 - How many hydrogen atoms are present in each...Ch. 10.5 - Prob. 10.13PCh. 10.5 - Prob. 10.14PCh. 10.5 - Prob. 10.15PCh. 10.5 - Prob. 10.16PCh. 10.6 - Give the IUPAC name for each compound.Ch. 10.6 - Prob. 10.18PCh. 10.6 - Prob. 10.19PCh. 10.6 - Prob. 10.20PCh. 10.7 - Prob. 10.21PCh. 10.7 - Prob. 10.22PCh. 10.9 - Answer the following questions about pentane...Ch. 10.9 - Prob. 10.24PCh. 10.9 - Prob. 10.25PCh. 10.10 - Prob. 10.26PCh. 10 - Prob. 10.27UKCCh. 10 - Prob. 10.28UKCCh. 10 - Prob. 10.29UKCCh. 10 - Prob. 10.30UKCCh. 10 - Prob. 10.31UKCCh. 10 - The largest known cycloalkane with a single ring...Ch. 10 - Draw three constitutional isomers having molecular...Ch. 10 - Draw four constitutional isomers having molecular...Ch. 10 - Answer the following questions about the alkane...Ch. 10 - Answer the questions in Problem 10.35 for the...Ch. 10 - Prob. 10.37UKCCh. 10 - Procaine (trade name Novocain) is a local...Ch. 10 - Prob. 10.39APCh. 10 - Prob. 10.40APCh. 10 - Complete each structure by filling in all Hs and...Ch. 10 - Prob. 10.42APCh. 10 - Prob. 10.43APCh. 10 - Prob. 10.44APCh. 10 - Prob. 10.45APCh. 10 - Prob. 10.46APCh. 10 - Convert each compound to a condensed structure.Ch. 10 - Prob. 10.48APCh. 10 - Prob. 10.49APCh. 10 - Prob. 10.50APCh. 10 - Prob. 10.51APCh. 10 - Prob. 10.52APCh. 10 - Albuterol (trade names: Proventil and Ventolin) is...Ch. 10 - Prob. 10.54APCh. 10 - Prob. 10.55APCh. 10 - Prob. 10.56APCh. 10 - Prob. 10.57APCh. 10 - Prob. 10.58APCh. 10 - Prob. 10.59APCh. 10 - Prob. 10.60APCh. 10 - Prob. 10.61APCh. 10 - Prob. 10.62APCh. 10 - Prob. 10.63APCh. 10 - Prob. 10.64APCh. 10 - Prob. 10.65APCh. 10 - Prob. 10.66APCh. 10 - Prob. 10.67APCh. 10 - Prob. 10.68APCh. 10 - Prob. 10.69APCh. 10 - Prob. 10.70APCh. 10 - Give the IUPAC name for each compound.Ch. 10 - Prob. 10.72APCh. 10 - Prob. 10.73APCh. 10 - Prob. 10.74APCh. 10 - Prob. 10.75APCh. 10 - Prob. 10.76APCh. 10 - Give the structure corresponding to each IUPAC...Ch. 10 - Prob. 10.78APCh. 10 - Prob. 10.79APCh. 10 - Each of the following IUPAC names is incorrect....Ch. 10 - Prob. 10.81APCh. 10 - Prob. 10.82APCh. 10 - Prob. 10.83APCh. 10 - Prob. 10.84APCh. 10 - Prob. 10.85APCh. 10 - Prob. 10.86APCh. 10 - Write a balanced equation for the incomplete...Ch. 10 - Prob. 10.88APCh. 10 - Prob. 10.89APCh. 10 - Prob. 10.90APCh. 10 - Prob. 10.91APCh. 10 - Prob. 10.92APCh. 10 - Answer the following questions for the cycloalkane...Ch. 10 - Prob. 10.94APCh. 10 - Prob. 10.95CPCh. 10 - Prob. 10.96CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) Write the structure of the indicated isomers. Give their IUPAC names. a. All possible chain isomers of (CH,), C b. Position isomers of cyclohexenol c. Functional isomer of acetophenone d. Functional isomer of butanethiol 2. Give the structure and IUPAC names of all possible skeletal isomers of 1-heptyne.arrow_forward9. Select the correct IUPAC name for : H,C- .CH, A. 1,4-dimethylcyclopentane. B. 1,3-dimethylcyclopentane. C. 2,5-dimethylcyclopentane. D. 2,3-dimethylcyclopentane. Agoula E. 2,4-dimethylcyclopentane. nobarrow_forward1. Draw the structure of each of the following cycloalkanes a. 1-Bromo-2-methylcyclobutaneb. 1,2-Dibromo-3-methylcyclohexanec. Iodocyclopropane2. How does the general formula of a cycloalkane compare to that of an alkane?arrow_forward
- 16. Draw condensed structures corresponding to each name below. a. 2-methyl-1,3-butadiene b. (2Z,4Z)-hexadienearrow_forwardBe sure to answer all parts. Draw the structure corresponding to each IUPAC name. a. 3,3-dimethylpentane CH₂ CH₂ edit structure... b. 3-ethyl-4-methylhexane -CH₂ CH₂ CH3 CH₂ edit structure ... CH₂ Xarrow_forwardWrite molecular formulas for each bicycloalkane, given its number of carbon atoms. Q.) Decalin (10 carbons)arrow_forward
- This is no multiple questions please answer only draw structure allarrow_forward1) Explain the similarities and differences between Lewis structures and line drawings. Provide a drawn example different from the one shown in the video. 2) Provide a summary of the IUPAC rules. Do not copy them verbatim from the lecture video. 3) Draw and name an alkane or cycloalkane that has 3 different substituents. You can not have the same example as someone else. 4) Draw and name an alkene that has an alkyne substituent. You can not have the same example as someone else. 5) Provide your own list of alkanes. They can not be the same structure but should all have the same number of carbons. (see the problem solving video for an example) Rank them in order of increasing melting point. You can not have the same example as someone else. MacBook Air DD F11 F9 F10 F8 F6 F7 F5 F4 F2 F3 ) & @ # $ % 4 5 6 7 8 9 2 3 { R Y [ + || 品。arrow_forward2. Identify the configuration of the alkene present in the compound. You will need to redraw the structure and insert your answer next to the carbon double bond. H. Brarrow_forward
- tudent Name: 7. Draw a bond-line structure of based on the following name. Determine if the systematic name provided is 9600 correct or incorrect. If the name is incorrect, provide a correct systematic name for the molecules. a. 2,2-dimethyl-4-ethylheptane XX nalda b. 5-butyl-3,3,9-trimethylundecane C. 3-isopropyloctane a 4-ethyl-2,2-dimethylheptane Lovel مین آماده همرا nchs husits, no no band a d. 1-methyl-3-sec-butylcyclohexane usu enollsmolno? $ tall at to do thought off والا بس الي الليل with amet gniwollot rose wala er to be anled died J-J planting * * bazgib S dan dras s thot ماء لامعة بومادر to meet to be as la actres des of Rank the alkanes in each group below in order of increasing boiling point (3 = lowest, 1 = highest). do not touted toarrow_forwardpls explain thanksarrow_forwardSome alkenes have cis-trans isomers because a. the carbon atoms in the double bond are free to rotate. b. one of the carbon atoms in the double bond has two identical groups attached to it. c. all of the carbon atoms in the compound are rigid and cannot rotate. d. each of the carbon atoms in the double bond has four different groups attached to it. e. the carbon atoms in the double bond cannot rotate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License