
a.
Interpretation:
The compounds butane or isobutane has to be represented.
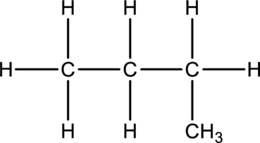
Concept Introduction:
Butane and Isobutane:
In butane structure there will be four carbon atoms present in the straight chain. The carbon present in one continuous chain.
In isobutane there will be three carbon atoms present in a row and one carbon bond is bonded to middle carbon. This
b.
Interpretation
The compounds whether butane or isobutane has to be identified.
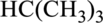
Concept Introduction:
Refer part: a.
c.
Interpretation:
The compounds either butane or isobutane has to be represented.
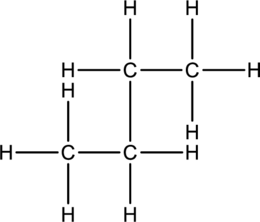
Concept Introduction:
Refer part: a.
d.
Interpretation:
The compounds butane or isobutane has to be represented.
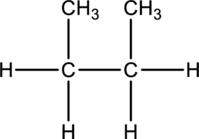
Concept-Introduction:
Refer part: a.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward
- State the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forward
- For the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardR lactam or lactone considering as weak acid or weak base and whyarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





