
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.4, Problem 10.7P
Identify the
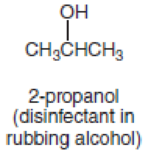
- a. H2NCH2CH2CH2CH2NH2
Putrescine (putrid odor of rotting fish)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Functional Group Classes
Alcohol
Amide
Ester
• CH₂CH₂OH
* CHIO-CH,
7. H₂C1O
Amine
Ketone
Circle each functional group(s) and identify the class to which each of the groups
belongs.
9. H₂C
Aldehyde
Thiol
Aromatics
Carboxylic Acid
2. CH,SH
6.
Alkenes
4. H₂NCH₂CH₂
10.
Ether
NM₂
11. Which of the functional groups in the top list must be on a terminalC in the carbon
chain?
what is the functional group involves?
1. Determine the IHD and circle and label the functional groups in each of the following compounds.
A. Caffeine (a stimulant and analgesic)
B. Naproxen (a NSAID)
N
N
-N
N
O
OH
Chapter 10 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 10.1 - Prob. 10.1PCh. 10.2 - Fill in all Hs and lone pairs in each compound. a....Ch. 10.3 - Convert each compound to a condensed formula.Ch. 10.3 - Convert each condensed formula to a complete...Ch. 10.3 - Convert each skeletal structure to a complete...Ch. 10.3 - How many Hs are bonded to each indicated carbon...Ch. 10.4 - Identify the functional groups in each compound....Ch. 10.4 - For each compound: [1] Identify the functional...Ch. 10.4 - Prob. 10.9PCh. 10.4 - Prob. 10.10P
Ch. 10.4 - Convert the ball-and-stick model of the local...Ch. 10.5 - How many hydrogen atoms are present in each...Ch. 10.5 - Prob. 10.13PCh. 10.5 - Prob. 10.14PCh. 10.5 - Prob. 10.15PCh. 10.5 - Prob. 10.16PCh. 10.6 - Give the IUPAC name for each compound.Ch. 10.6 - Prob. 10.18PCh. 10.6 - Prob. 10.19PCh. 10.6 - Prob. 10.20PCh. 10.7 - Prob. 10.21PCh. 10.7 - Prob. 10.22PCh. 10.9 - Answer the following questions about pentane...Ch. 10.9 - Prob. 10.24PCh. 10.9 - Prob. 10.25PCh. 10.10 - Prob. 10.26PCh. 10 - Prob. 10.27UKCCh. 10 - Prob. 10.28UKCCh. 10 - Prob. 10.29UKCCh. 10 - Prob. 10.30UKCCh. 10 - Prob. 10.31UKCCh. 10 - The largest known cycloalkane with a single ring...Ch. 10 - Draw three constitutional isomers having molecular...Ch. 10 - Draw four constitutional isomers having molecular...Ch. 10 - Answer the following questions about the alkane...Ch. 10 - Answer the questions in Problem 10.35 for the...Ch. 10 - Prob. 10.37UKCCh. 10 - Procaine (trade name Novocain) is a local...Ch. 10 - Prob. 10.39APCh. 10 - Prob. 10.40APCh. 10 - Complete each structure by filling in all Hs and...Ch. 10 - Prob. 10.42APCh. 10 - Prob. 10.43APCh. 10 - Prob. 10.44APCh. 10 - Prob. 10.45APCh. 10 - Prob. 10.46APCh. 10 - Convert each compound to a condensed structure.Ch. 10 - Prob. 10.48APCh. 10 - Prob. 10.49APCh. 10 - Prob. 10.50APCh. 10 - Prob. 10.51APCh. 10 - Prob. 10.52APCh. 10 - Albuterol (trade names: Proventil and Ventolin) is...Ch. 10 - Prob. 10.54APCh. 10 - Prob. 10.55APCh. 10 - Prob. 10.56APCh. 10 - Prob. 10.57APCh. 10 - Prob. 10.58APCh. 10 - Prob. 10.59APCh. 10 - Prob. 10.60APCh. 10 - Prob. 10.61APCh. 10 - Prob. 10.62APCh. 10 - Prob. 10.63APCh. 10 - Prob. 10.64APCh. 10 - Prob. 10.65APCh. 10 - Prob. 10.66APCh. 10 - Prob. 10.67APCh. 10 - Prob. 10.68APCh. 10 - Prob. 10.69APCh. 10 - Prob. 10.70APCh. 10 - Give the IUPAC name for each compound.Ch. 10 - Prob. 10.72APCh. 10 - Prob. 10.73APCh. 10 - Prob. 10.74APCh. 10 - Prob. 10.75APCh. 10 - Prob. 10.76APCh. 10 - Give the structure corresponding to each IUPAC...Ch. 10 - Prob. 10.78APCh. 10 - Prob. 10.79APCh. 10 - Each of the following IUPAC names is incorrect....Ch. 10 - Prob. 10.81APCh. 10 - Prob. 10.82APCh. 10 - Prob. 10.83APCh. 10 - Prob. 10.84APCh. 10 - Prob. 10.85APCh. 10 - Prob. 10.86APCh. 10 - Write a balanced equation for the incomplete...Ch. 10 - Prob. 10.88APCh. 10 - Prob. 10.89APCh. 10 - Prob. 10.90APCh. 10 - Prob. 10.91APCh. 10 - Prob. 10.92APCh. 10 - Answer the following questions for the cycloalkane...Ch. 10 - Prob. 10.94APCh. 10 - Prob. 10.95CPCh. 10 - Prob. 10.96CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Encircle the functional groups, label each functional group with a letter, and identify the type or class of compounds represented by each functional group. 1. LSD, a hallucinogenic drug widely believed to be the inspiration behind the Beatles hit “Lucy in the Sky with Diamonds” 2. Methyljasmonate, a compound that belongs to a group called the pheromones. The male oriental fruit moth, Grapholitha molesta Busk., responds when a female moth emits this compound. 3.arrow_forwardin medicine as the first line of defence against breast cancer. Identify ALL the different functional groups in the molecule. оно NH ÖH O A. Aromatic ring, carboxylic acid, ester, amide, alkene, alcohol, cyclic ether. B. Substituted benzene ring, amide, alcohol, ester, alkene, ketone, cyclic ether. C. Substituted benzene ring, carboxylic acid, ester, amide, alkene, alcohol, aldehyde. D. Aromatic ring, ketone, cyclic ester, amide, alkene, alcohol, aldehyde. E. Aromatic ring, aldehyde, ester, amide, alkene, alcohol, acyclic ether.arrow_forwardhelp naming/classifying compoundsarrow_forward
- Q1 Aldehydes contain the carbonyl group bonded to at least one hydrogen atom. contain the carbonyl group bonded to two carbon atoms contain the carbonyl group bonded to three carbon atoms Q2 compounds with the general formula R S. R′, where R and R′ are hydrocarbon radicals. These compounds can be considered as analogs of ethers, generated by replacing the oxygen atom with sulfu. Organic sulfides C16H32 C16H34 Q3 CH3 CH2 OH 1)Ethyl alcohol 2)2- methyl propanol 3)propyl Q5 The boiling point increase with increasing carbon number , and they usually decrease with branching . 1)Alcohols 2)Ethers 3)Di ethyl ether Q4 The first three primary alcohols soluble in water 1)T 2)F Q6 organic compounds which incorporate a carbonyl functional group, C=O. 1)Aldehydes and ketones 2)Phenol 3)alcohols Q7 Crude oil 1)is a mixture of hydrocarbons 2)Option 2 3)Option 3 Q8 types of hydrocarbon compounds present in crude oil 1)paraffins 2)naphthenes 3)ALL ITQ11 The hydrogen…arrow_forwardIdentify the functional group in each compound. Part 1 of 2arrow_forward1. Which of the following statements is true? I. Aldehydes and ketones both contain a hydroxyl group. II. The names for aldehydes and ketones are derived from the name of the longest carbon chain that contains the carbonyl group. III. The aldehyde and ketone with a molecular formula of C3H6O are constitutional isomers. IV. 2-Propanone is immiscible in water. A. I & II B. II & II C. I & III D. I & IV 2. Whicb of the following is the correct IUPAC name of the structure below?arrow_forward
- Functionalized Hydrocarbons Identify each compound according to its functional group (e.g.,amine,ester,etc.):arrow_forward16. An atom or group of atoms that can give organic compounds distinct chemical and physical properties. 21. When a compound with the general formula R-COOH loses a proton, the product that remains is described with this term. Its general formula is R-COO- 24. A class of organic compounds in which three or more carbons form a ring structure. All of the carbon-to-carbon bonds are single bonds in this family of compounds.arrow_forwardIdentify the functional group present - Functional Group Earrow_forward
- Choose the correct functional group present in each structure below that would dictate how it would be named. Functional Group Identify type [ Select ] R. carboxylic acid R. ОН R. amide `NH2 R. [ Select ] `OH [ Select ] R' R. [ Select ]arrow_forwardFUNCTIONAL GROUP IDENTIFICATION WORKSHEET 1. Identify the functional groups on the following organic molecules. а. H. OH H H b. H- H нн H H H-C C C-Ć-H нн нн d. H. OH H -- H. Br H -C H H. C. e.arrow_forwardZanamivir is an antiviral drug. Identify and circle each functional group (write for example primary amine, secondary alcohol, aldehyde, alkyne, etc.).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY