
Concept explainers
a.
Interpretation:
The hydrogen and lone pairs of electrons of given compound has to be drawn.
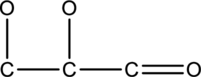
Concept Introduction:
BOND LINE STRUCTURE:
The bond line structure is a simple way of representing a structure of carbon compound. Bonding of atom is represented by lines.
LEWIS STRUCTURE:
The bonds have to be drawn between atoms and the lone pair of electron present in a compound. It is otherwise known as Lewis dot diagram.
OCTET RULE:
The atoms share or lose or gain electrons to satisfy nearest electronic configuration of noble gases. The compound should be surrounded by eight valence electrons to form octet.
b.
Interpretation:
The hydrogen and lone pairs of given compound has to be drawn.
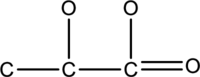
Concept Introduction:
Refer part: a.
c.
Interpretation:
The hydrogen and lone pairs of given compound has to be observed.
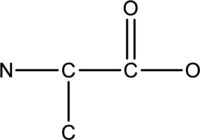
Concept-Introduction:
Refer part: a.
d.
Interpretation:
The given compound in which hydrogen and lone pairs has to be drawn.
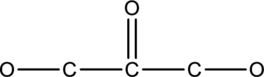
Concept-Introduction:
Refer part: a.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





