
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10.5, Problem 10.14P
a.
Interpretation Introduction
Interpretation:
The given compounds whether it is constitutional isomer or not has to be identified.
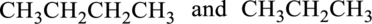
Concept Introduction:
Isomers:
Isomers are defined as same molecular formula and different structural formula.
Constitutional Isomer:
Constitutional isomer has same molecular formula but they have different connectivity to each other. It is also known as structural isomers.
b.
Interpretation Introduction
Interpretation
The given compound either constitutional isomer or not has to be represented.
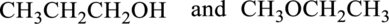
Concept Introduction:
Refer part a.
c.
Interpretation Introduction
Interpretation:
The given compound is constitutional isomer or not has to be found.
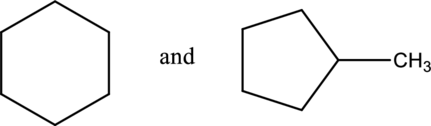
Concept Introduction:
Refer part a.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic.
НОН НЬ
OHd
Онс
Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left?
?
starting
material
target
If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area.
Be sure you follow the standard ALEKS rules for submitting syntheses.
+ More...
Note for advanced students: you may assume that you are using a large excess of benzene as your starting material.
C
:0
T
Add/Remove step
G
The following equations represent the formation of compound MX. What is the AH for the
electron affinity of X (g)?
X₂ (g) → 2X (g)
M (s) → M (g)
M (g)
M (g) + e-
AH = 60 kJ/mol
AH = 22 kJ/mol
X (g) + e-X (g)
M* (g) +X (g) → MX (s)
AH = 118 kJ/mol
AH = ?
AH = -190 kJ/mol
AH = -100 kJ/mol
a)
-80 kJ
b)
-30 kJ
c)
-20 kJ
d)
20 kJ
e)
156 kJ
Chapter 10 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 10.1 - Prob. 10.1PCh. 10.2 - Fill in all Hs and lone pairs in each compound. a....Ch. 10.3 - Convert each compound to a condensed formula.Ch. 10.3 - Convert each condensed formula to a complete...Ch. 10.3 - Convert each skeletal structure to a complete...Ch. 10.3 - How many Hs are bonded to each indicated carbon...Ch. 10.4 - Identify the functional groups in each compound....Ch. 10.4 - For each compound: [1] Identify the functional...Ch. 10.4 - Prob. 10.9PCh. 10.4 - Prob. 10.10P
Ch. 10.4 - Convert the ball-and-stick model of the local...Ch. 10.5 - How many hydrogen atoms are present in each...Ch. 10.5 - Prob. 10.13PCh. 10.5 - Prob. 10.14PCh. 10.5 - Prob. 10.15PCh. 10.5 - Prob. 10.16PCh. 10.6 - Give the IUPAC name for each compound.Ch. 10.6 - Prob. 10.18PCh. 10.6 - Prob. 10.19PCh. 10.6 - Prob. 10.20PCh. 10.7 - Prob. 10.21PCh. 10.7 - Prob. 10.22PCh. 10.9 - Answer the following questions about pentane...Ch. 10.9 - Prob. 10.24PCh. 10.9 - Prob. 10.25PCh. 10.10 - Prob. 10.26PCh. 10 - Prob. 10.27UKCCh. 10 - Prob. 10.28UKCCh. 10 - Prob. 10.29UKCCh. 10 - Prob. 10.30UKCCh. 10 - Prob. 10.31UKCCh. 10 - The largest known cycloalkane with a single ring...Ch. 10 - Draw three constitutional isomers having molecular...Ch. 10 - Draw four constitutional isomers having molecular...Ch. 10 - Answer the following questions about the alkane...Ch. 10 - Answer the questions in Problem 10.35 for the...Ch. 10 - Prob. 10.37UKCCh. 10 - Procaine (trade name Novocain) is a local...Ch. 10 - Prob. 10.39APCh. 10 - Prob. 10.40APCh. 10 - Complete each structure by filling in all Hs and...Ch. 10 - Prob. 10.42APCh. 10 - Prob. 10.43APCh. 10 - Prob. 10.44APCh. 10 - Prob. 10.45APCh. 10 - Prob. 10.46APCh. 10 - Convert each compound to a condensed structure.Ch. 10 - Prob. 10.48APCh. 10 - Prob. 10.49APCh. 10 - Prob. 10.50APCh. 10 - Prob. 10.51APCh. 10 - Prob. 10.52APCh. 10 - Albuterol (trade names: Proventil and Ventolin) is...Ch. 10 - Prob. 10.54APCh. 10 - Prob. 10.55APCh. 10 - Prob. 10.56APCh. 10 - Prob. 10.57APCh. 10 - Prob. 10.58APCh. 10 - Prob. 10.59APCh. 10 - Prob. 10.60APCh. 10 - Prob. 10.61APCh. 10 - Prob. 10.62APCh. 10 - Prob. 10.63APCh. 10 - Prob. 10.64APCh. 10 - Prob. 10.65APCh. 10 - Prob. 10.66APCh. 10 - Prob. 10.67APCh. 10 - Prob. 10.68APCh. 10 - Prob. 10.69APCh. 10 - Prob. 10.70APCh. 10 - Give the IUPAC name for each compound.Ch. 10 - Prob. 10.72APCh. 10 - Prob. 10.73APCh. 10 - Prob. 10.74APCh. 10 - Prob. 10.75APCh. 10 - Prob. 10.76APCh. 10 - Give the structure corresponding to each IUPAC...Ch. 10 - Prob. 10.78APCh. 10 - Prob. 10.79APCh. 10 - Each of the following IUPAC names is incorrect....Ch. 10 - Prob. 10.81APCh. 10 - Prob. 10.82APCh. 10 - Prob. 10.83APCh. 10 - Prob. 10.84APCh. 10 - Prob. 10.85APCh. 10 - Prob. 10.86APCh. 10 - Write a balanced equation for the incomplete...Ch. 10 - Prob. 10.88APCh. 10 - Prob. 10.89APCh. 10 - Prob. 10.90APCh. 10 - Prob. 10.91APCh. 10 - Prob. 10.92APCh. 10 - Answer the following questions for the cycloalkane...Ch. 10 - Prob. 10.94APCh. 10 - Prob. 10.95CPCh. 10 - Prob. 10.96CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY