
(a)
Interpretation:
The composition of the solid phase and the liquid phase in mol% and wt% are to be calculated for
Concept Introduction:
On the temperature-composition graph of a ceramic, the curve above which the ceramic exist in the liquid phase is the liquidus curve. The temperature at this curve is the maximum temperature at which the crystals in the ceramic can coexist with its melt in the
Solidus curve is the locus of the temperature on the temperature composition graph of a ceramic, beyond which the ceramic is completely in solid phase.
Between the solidus and liquidus curve, the ceramic exits in a slurry form in which there is both crystals as well as ceramic melt.
Solidus temperature is always less than or equal to the liquidus temperature.
The formula to calculate the wt% from the mol% for a ceramic containing phases
Here,
Answer to Problem 10.53P
Composition of the liquid phase in mol% is
Composition of the liquid phase in wt% is
Composition of the solid phase in mol% is
Composition of the solid phase in wt% is
Explanation of Solution
The phase diagram for NiO-MgO is given as:
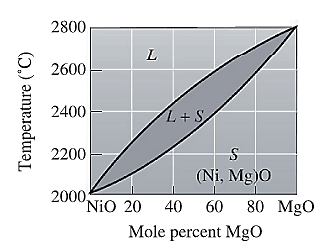
Now, draw a straight line from temperature
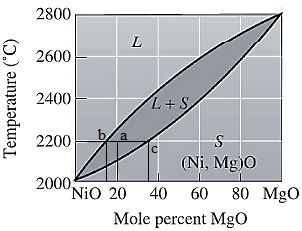
Here, point 'a' represents
Molecular weight of NiO and MgO are
Use equation (1) to convert mol% to wt% for liquid phase as:
Again, use equation (1) to convert mol% to wt% for solid phase as:
(b)
Interpretation:
The amount of each phase present in
Concept Introduction:
A matter can exist in different physical forms such as sold, liquid, gas, and plasma. These distinct physical forms are known as a Phase.
A phase has uniform physical and chemical properties and is bounded by a surface due to which two phases can be
The formula to calculate the wt% from the mol% for a ceramic containing phases
Here,
Amount of each phase in mol% is calculated using lever rule. At a particular temperature and ceramic composition, a tie line is drawn on the phase diagram of the ceramic between the solidus and liquidus curve. Then the portion of the lever opposite to the phase whose amount is to be calculated is considered in the formula used as:
Answer to Problem 10.53P
Amount of liquid phase in mol% is
Amount of liquid phase in wt% is
Amount of solid phase in mol% is
Amount of solid phase in wt% is
Explanation of Solution
The phase diagram for NiO-MgO is given as:
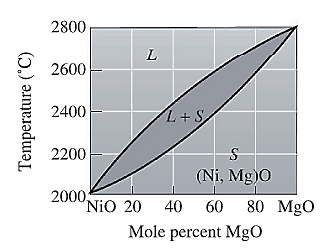
Now, draw a straight line from temperature
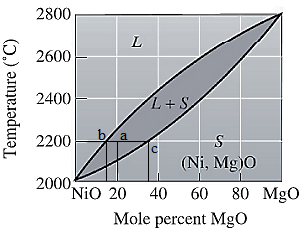
Here, point 'a' represents
To calculate amount of liquid phase, lever 'ac' will be used and to calculate amount of solid phase, lever 'ba' will be used. Use equation (2) to calculate the amount of each phase as:
To calculate the amount of liquid and solid phases in wt%, first convert the original mol% of MgO in wt% using equation (1) and molecular weights of NiO and MgO as:
To apply the lever rule, use the corresponding wt% for the liquid and solid phases as calculated in part (a) as:
Apply lever rule as:
(c)
Interpretation:
The amount of each phase is to be calculated in vol%.
Concept Introduction:
The formula to convert wt% to vol% using density
Answer to Problem 10.53P
The amount of liquid phase in vol% is
The amount of solid phase in vol% is
Explanation of Solution
Given information:
A ceramic containing
From part (b), the amount of liquid and solid phases in wt% is calculated as:
Use equation (3) along with the given densities of the phases to calculate the vol% as:
Want to see more full solutions like this?
Chapter 10 Solutions
Essentials Of Materials Science And Engineering
- The MATLAB code is going well but the last part in bandpass, the legend that is supposed to tell the color of both lower and upper-frequency cutoff does not align with each other. As such I need help My Matlab code: % Define frequency range for the plot f = logspace(1, 5, 500); % Frequency range from 10 Hz to 100 kHz w = 2 * pi * f; % Angular frequency % Parameters for the filters R = 1e3; % Resistance in ohms (1 kΩ) C = 1e-6; % Capacitance in farads (1 μF) L = 0.1; % Inductance in henries (chosen for proper bandpass response) % Compute cutoff frequencies f_cutoff_RC = 1 / (2 * pi * R * C); % RC low-pass/high-pass cutoff f_resonance = 1 / (2 * pi * sqrt(L * C)); % Resonant frequency of RLC Q_factor = (1/R) * sqrt(L/C); % Quality factor of the circuit % Band-pass filter cutoff frequencies f_lower_cutoff = f_resonance / (sqrt(1 + 1/(4*Q_factor^2)) + 1/(2*Q_factor)); f_upper_cutoff = f_resonance / (sqrt(1 + 1/(4*Q_factor^2)) - 1/(2*Q_factor)); % Define Transfer Functions H_low =…arrow_forwardWhich of the following opens when you click the launcher in the Size group on the Ribbon? Question 19Select one: a. Size dialog box b. Layout dialog box c. Width and Height dialog box d. Format dialog boxarrow_forwardHow do you resize a graphic object horizontally while keeping the center position fixed? Question 20Select one: a. Drag a side sizing handle. b. Press [Ctrl] and drag a side sizing handle. c. Press [Alt] and drag a side sizing handle. d. Press [Shift] and drag a side sizing handle.arrow_forward
- Which of the following indicates that a graphic is anchored to the nearest paragraph? Question 18Select one: a. X and Y coordinates b. An anchor symbol c. A paragraph symbol d. ruler marksarrow_forwardWhich command in the Adjust group allows you to change one picture for another but retain the original picture's size and formatting? Question 17Select one: a. Change Picture b. Replace c. Swap d. Relinkarrow_forwardPost-tensioned AASHTO Type III girders are to be used to support a deck with unsupported span equal to 90 feet. One level of Grade 270, 10 x 0.6" Ø 7-wire strand are used to tension the girders. The girder is simply supported at both ends. The anchors are located 2" from the neutral axis at the supports while the eccentricity is measured at 14" at the midspan. Use maximum values for ranges (table values). The tendons are encased in flexible metal sheathing. Assume and loadings are placed immediately after stressing. Determine the stresses at the top and bottom of the beam, including the stress at the level of steel after three years. Given the following parameters: F'c = 5000 psi Fy = 240 ksi (Bonded, Stress-relieved) Fu = 270 ksi = Es 29,000 ksi Ec = 4,030 psi Fj = 235 ksi A = 1x0.6" 07-W.S. = 0.217 in² Yc = 140 lbs/ft³ ΔΑ = 3/8" RH = 33% Superimposed Service Load = 10 kips/ft (excluding self-weight)arrow_forward
- How do you insert multiple rows at the same time? Question 10Select one: a. Select the number of rows you want to insert, then use an Insert Control or use the buttons on the Ribbon. b. Click Insert Multiple Rows in the Rows & Columns group. c. Select one row and click the Insert Above or Insert Below button. You will be prompted to choose how many rows to insert. d. You cannot insert multiple rows at the same time.arrow_forwardHow do you center the text vertically in each table cell? Question 9Select one: a. Select the table and click the Distribute Columns button. b. Select the table and click the Center button in the Paragraph group on the Home tab. c. Select the table and click the AutoFit button. d. Click the Select button in the Table group, click Select Table, then click the Align Center Left button in the Alignment group.arrow_forwardA(n) ____ is a box formed by the intersection of a column and a row. Question 8Select one: a. divider b. table c. border d. cellarrow_forward
- A ____ row is the first row of a table that contains the column headings. Question 7Select one: a. header b. primary c. title d. headingarrow_forwardFIG. P5.66 40 kN B 4 m A 20 kN 10 kN/m C 8 m 8 marrow_forwardCase study: Multiple customers have launched complaints that searching for and purchasing music from Sonic Selection is confusing and laborious. The management executives recognise this as a major problem, as they pride themselves on putting their customers first. Therefore, they decide that the IT team should update the user interface, and streamline their platforms to make them more user-friendly. Before the IT team can redesign the platform, the company decides to do in-depth research into the problem, in order to guide the web designers. Question 1 Formulate three specific research questions that the company could propose in order to guide the IT team during the redesign process. Formulate one descriptive, one relational, and one causal question. For each question, clearly state whether it is a descriptive, relational, or causal question. Be sure to apply the research question best practice guidelines highlighted in this unit’s notes to ensure that the questions are…arrow_forward
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





