
(a)
Interpretation:
The element having the given configuration has to be identified. Also whether the configuration represent ground state or not has to be identified.
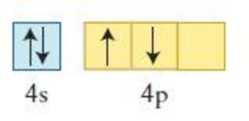
Figure 1
Concept Introduction:
Electronic configuration: The electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
Electrons occupy the lowest energy orbitals. The increasing order of orbital energy is
The energy order of the orbital for the first three periods is as follows,
The orbital which is closer to the nucleus has lower energy; therefore the
In general, the orbitals can hold maximum of two electrons, the two electrons must have opposite spin.
The subshell ordering by Aufbau principle is given below,
Electrons are filled in each orbital one after another in the increasing order of energy. While filling the orbitals, more than two electrons can be placed in an orbital. And also, the spin of the two electron in the same orbital must be paired.
If there are more than one orbitals in a subshell are available for filling the electron, then electrons with parallel spin goes to different subshell rather than pairing two electrons in one orbital.
If an atom having electrons in energy states higher than predicted by the above rules then it is said to be in an excited state.
(b)
Interpretation:
The element having the given configuration has to be identified. Also whether the configuration represent ground state or not has to be identified.
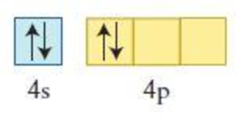
Figure 2
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The element having the given configuration has to be identified. Also whether the configuration represent ground state or not has to be identified.
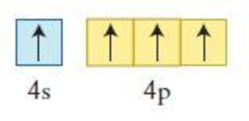
Figure 3
Concept Introduction:
Refer to part (a).
(d)
Interpretation:
The element having the given configuration has to be identified. Also whether the configuration represent ground state or not has to be identified.
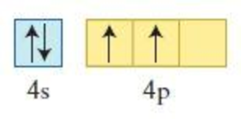
Figure 4
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
CHEMICAL PRINCIPLES (LL) W/ACCESS
- I have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forwardFirst image: Why can't the molecule C be formed in those conditions Second image: Synthesis for lactone C its not an examarrow_forward
- First image: I have to show the mecanism for the reaction on the left, where the alcohol A is added fast in one portion Second image: I have to show the mecanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primaryarrow_forwardFirst image: I have to explain why the molecule C is never formed in those conditions. Second image: I have to propose a synthesis for the lactone Aarrow_forwardFirst image: I have to explain why the molecule C is never formed in these conditions Second image: I have to propose a synthesis for the lactone Aarrow_forward
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





